An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Meditation and the wandering mind: a theoretical framework of underlying neurocognitive mechanisms
Tracy brandmeyer.
1 Osher Center for Integrative Medicine, School of Medicine, University of California, San Francisco, 94115, USA
2 Centre de Recherche Cerveau et Cognition (CerCo), Université Paul Sabatier, Toulouse, France
3 CNRS, UMR 5549, Toulouse, France
Arnaud Delorme
4 Swartz Center for Computational Neuroscience, Institute of Neural Computation (INC), University of California, San Diego, La Jolla 92093-0559, USA
During the practice of meditation, the occurrence of self-generated and spontaneous thought and the tendency of the mind to wander away from the intended goal of meditation is ubiquitous, and comprises one of the fundamental teachings of meditation: a heightened awareness of where our attention is placed, and an awareness of the contents of our mind. Mind-wandering in the context of meditation provides individuals a unique and intimate opportunity to closely examine the nature of the wandering mind, cultivating an awareness of ongoing thought patterns while simultaneously cultivating equanimity (evenness of temper or disposition) and compassion towards the content of thoughts, interpretations, and bodily sensations. In this review we provide a theoretical framework highlighting the neurocognitive mechanisms by which contemplative practices influence the neural and phenomenological processes underlying spontaneous thought. Our theoretical model focuses on several converging mechanisms: the role of meta-awareness in facilitating an increased moment to moment awareness of spontaneous thought processes, the effects of meditation practice on key structures underlying both the top-down cognitive processes and bottom-up sensory processes implicated in attention and emotion regulation, and the influence of contemplative practice on the neural substrates underlying perception and perceptual decoupling.
Introduction
Over last few decades we have witnessed an exponential rise of scientific interest and research on the effects of meditation practices and mindfulness-based interventions on brain structure and function ( Fox et al., 2014 ; Lazar et al., 2005 ), behavior ( Flook, Goldberg, Pinger, & Davidson, 2015 ; Malouf, Youman, Stuewig, Witt, & Tangney, 2017 ; Singh, Lancioni, Wahler, Winton, & Singh, 2008 ), genetic expression ( Epel et al., 2013 ; Ornish et al., 2013 ), medical outcomes ( Morone, Greco, & Weiner, 2008 ; Rosenzweig et al., 2010 ), clinical outcomes ( Goyal et al., 2014 ), military performance ( Carter & Carter III, 2016 ), professional performance ( McConville, McAleer, & Hahne, 2017 ) and more. Accumulating research findings provide compelling evidence that mind-body practices such as meditation are associated with improvements in cognitive and affective processes ( Tang, Hölzel, & Posner, 2015 ). Meditation practices aim to generate a dispositional quality refered to as mindfulness, which is considered to be a self-regulated attentional state focused on present-moment experience, emphasizing the features of curiosity, openness, and acceptance ( Dahl, Lutz, & Davidson, 2015 ; Kabat-Zinn, 2003 ). While a majority of meditation practices aim to generate mindfulness through techniques that cultivate attentional clarity and stability ( Wallace, 1999 ), regulate emotional responses to the content of our thoughts and experiences ( Teper, Segal, & Inzlicht, 2013 ), and cultivate compassion for oneself and others ( Hofmann, Grossman, & Hinton, 2011 ), the precise definition and translation of the term mindfulness (Pali: sati or Sanskrit: smrti) is currently a heavily debated topic among Buddhist scholars and scientists (Kirk Warren Brown & Ryan, 2004 ; Fox et al., 2014 ). Given that 33.2% of U.S. adults report the use of mindfulness, meditation, or some form of complementary health approach or intervention ( Okoro, Zhao, Li, & Balluz, 2012 ), there is an increasing need for scientific research that sets forth clear terminology and constructs, elucidates the specific mechanisms by which meditation and mindfulness based practices and interventions exert their influence, and provides context as to how they may be optimally tailored to meet individual needs.
In parallel with the rise in popularity of contemplative science research (as well as the revival of methodologies that study the phenomenology of internal experience), the independent study of self-generated thought and the underlying psychological and neural mechanisms has become a key aim of cognitive neuroscientific research in recent years ( Christoff, Irving, Fox, Spreng, & Andrews-Hanna, 2016 ; Fox et al., 2018 ). Self-generated thought constitutes a diverse and complex class of cognition which generally refers to mental content that occurs largely independent of the external environment, forming a stream of thoughts that can include memories, future plans, daydreams and fantasies, mental imagery, simulated social interactions, rumination, dreams, and more ( Christoff et al., 2016 ). Like mindfulness, the scientific definition of self-generated thought and its corresponding phenomenology have been a recent subject of debate, with refinements in the categorical delineations between various forms of self-generated thought evolving alongside accumulating neuroimaging data. A recently proposed ‘state space’ framework developed by Christoff et al., 2016 highlights how both deliberate (i.e., intentional, top-down) and automatic (i.e., unconscious, bottom-up) constraints influence the content of self-generated thought ( Fig. 1 ). According to this framework, self-generated thoughts include spontaneous (unconstrained and unintentional) forms of cognition like dreaming, mind-wandering, and creative thinking ( Baird et al., 2012 ; Schooler et al., 2014 ), but also include forms of cognition such as rumination, obsessive thinking, and other habitual or automatically constrained patterns of thought ( Mrazek, Smallwood, & Schooler, 2012 ; Unsworth & McMillan, 2013 ). Denoted by their independence from external stimuli, spontaneous thought, a term commonly interchanged with the broader term mind-wandering in the scientific literature and throughout this review ( Andrews-Hanna, Irving, Fox, Spreng, & Christoff, 2017 ), can be understood as a mental state or sequence of mental states that arise relatively freely due to an absence of strong constraints on the contents of each state, and on the transitions from one mental state to another, lacking strong deliberate control ( Christoff et al., 2016 ). General estimates suggest that individuals engage in some form of self-generated thought approximately 30–50% of waking hours ( Killingsworth & Gilbert, 2010 ). Although cognitive psychologists and researchers have been investigating the specific mechanisms underlying spontaneous thought and mind-wandering for several decades now ( Singer, 1966 ), advancements in research methodologies and scientific rigor over the last several years has allowed for a more nuanced neurophenomenological understanding of internal experience ( Andrews-Hanna, Smallwood, & Spreng, 2014 ; Fox et al., 2014 ; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015 ; J. Smallwood & Andrews-Hanna, 2013 ).

The states space framework by Christoff et al., (2016) proposes a «conceptual space relating the concept of self-generated thought to deliberate and automatic constraints on cognition. Self-generated thought, by which we mean all those types of thought that are relatively independent of the external environment and immediate sensory inputs, spans a broad cognitive state space. Within this cognitive state space, both deliberate (intentional, top-down) and automatic (unconscious, bottom-up) constraints can influence the content of thought. “Spontaneous” thought is not only self-generated, but is also specifically characterized by relatively weak deliberate and automatic constraints. Rumination and obsessive thought are likewise self-generated and low in deliberate constraints, but are characterized by strong automatic constraints». Used with permission from Christoff et al., (2016) .
These increasingly refined methodological approaches applied within the domains of self-generated thought and contemplative science ( Bockelman, Reinerman-Jones, & Gallagher, 2013 ; Lutz & Thompson, 2003 ; Petitmengin & Lachaux, 2013 ; Thompson, 2008 ) have led to considerable progress in characterizing the first-person phenomenological experience and the associated neural correlates (Stawarczyk, 2018). Although the relevance of studying internal experience has garnered ample attention over the last decade, detailed phenomenological descriptions of the benefits of meditation, as well as the impact of mind-wandering on well-being, have been documented in scholarly Buddhists texts dating back over two thousand years (Santi-deva, 1961). Throughout these ancient writings an emphasis is placed on how attention, when it is not trained, becomes habitually prone to mind-wandering, agitation, and vapidity ( Wallace, 1999 ), a mental state referred to as the ‘monkey mind’ (Gunaratana, 2010). During meditation practice the tendency of the mind to wander away from the intended object is ubiquitous (unless the meditation practice that does not include any specific object or target of attention), and its occurrence can be used as means of cultivating an awareness of its frequency, duration, and content, in order to gain insight into the nature of one’s thoughts and to facilitate the stability of attention. As meditation practice inherently cultivates an ability to monitor cognitive processes related to attention and distraction, meditation practitioners are particularly well suited to report on the phenomenological nature of these mental events, with accumulating evidence suggesting that meditation expertise increases the accuracy and reliability of first-person reporting and interoceptive acuity ( Brandmeyer & Delorme, 2018 ; Fox et al., 2012 ).
Here we aim to further a dialogue regarding the convergence between historically separate lines of research ( Mrazek et al., 2012 ) by providing a broad theoretical framework highlighting the key neurocognitive mechanisms by which contemplative practice may influence the underlying mechanisms mediating spontaneous thought processes. We start by exploring the wealth of literature supporting the relationship between contemplative practice and the manner by which the meditation training inherently cultivates a meta-awareness of the wandering mind. We specifically highlight the influence of contemplative practice on emotional reactivity and the reappraisal of spontaneous thoughts as key mechanisms by which meditation training influences the associated cognitive dynamics. We then delve into a more granular explication of spontaneous thought and mind-wandering, highlighting the intricacies of its phenomenology and its occurrence during contemplative practice. Subsequently we synthesize research findings to illustrate how spontaneous thought processes may be mediated by brain networks functionally and structurally linked to the practice of meditation. We then review the extensive literature on the role of a brain network known as the default network (DMN; Raichle et al., 2001 ) in both mind-wandering and meditation, and explore recent findings implicating distributed brain networks beyond the DMN. Lastly, we present a synthesis of findings highlighting the mechanisms by which meditation modulates attention and sensory perception, and the mechanisms underlying perceptual coupling and decoupling implicated in spontaneous thought processes. Throughout this manuscript we highlight the multidirectional relationship between these key mechanisms, and conclude with a section on perspectives, future directions, and the translational applications of this framework.
Meditation: cultivating meta-awareness and equanimity with the wandering mind
Scientific interest in the neurophysiological bases of meditation has in large part come from our understanding of neuroplasticity and various forms of experience-induced changes that occur in the brain ( Lutz, Slagter, Dunne, & Davidson, 2008 ). The regular practice of meditation has been associated with increased functional connectivity ( Farb et al., 2007 ; Froeliger et al., 2012a ; Garrison, Scheinost, Constable, & Brewer, 2014 ; Garrison et al., 2014 ; Hasenkamp & Barsalou, 2012 ; Taren et al., 2015 ) as well as structural changes in the brain ( Hofmann et al., 2011 ; Hölzel et al., 2011 ; Lazar et al., 2005 ). Lazar and colleagues (2005) were the first to show that the prefrontal cortex and right anterior insula, regions heavily implicated in attention monitoring and regulation ( Lutz et al., 2008 ; Menon & Uddin, 2010 ; Tang & Posner, 2009 ), self-generated and spontaneous thought processes ( Christoff, Ream, Geddes, & Gabrieli, 2003 ; Fox et al., 2018 ; Vanhaudenhuyse et al., 2011 ), interoception ( Craig, 2002 ; Khalsa et al., 2008 ), and sensory processing ( Haegens, Osipova, Oostenveld, & Jensen, 2010 ; Kerr et al., 2011 ; Kerr, Sacchet, Lazar, Moore, & Jones, 2013 ), were thicker in experienced meditation practitioners than in age and gender matched controls. They also found that the between-group differences in prefrontal cortical thickness were most pronounced in older practitioners, suggesting that meditation may slow age-related cortical thinning and that the thickness of these two specific areas also correlated with the degree of meditation experience. Lazar and colleagues provided some of the first structural evidence for experience-dependent cortical plasticity associated with meditation practice ( Lazar et al., 2005 ). Additional research implicating the prefrontal cortex during focused attention meditation comes from the findings of Hasenkamp and colleagues (2012). The study highlights the naturalistic cognitive fluctuations between mind-wandering and attentional states derived from the practice of focused attention meditation. Their model proposes key intervals in the cognitive cycle of focused meditation: mind-wandering, awareness of mind-wandering, shifting of attention, and sustained attention and provides a foundation for the theoretical framework we discuss throughout this manuscript ( Hasenkamp, Wilson-Mendenhall, Duncan, & Barsalou, 2012 ). Their findings support and extend theories regarding the central role of mind-wandering and its detection during focused meditation, as well as the cognitive correlates of distributed brain networks.
Despite the central role of mind-wandering and its occurrence during meditation ( Banks, Welhaf, & Srour, 2015 ; Brandmeyer & Delorme, 2018 ; Evans & Segerstrom, 2011 ; Frewen, Evans, Maraj, Dozois, & Partridge, 2008 ; Hasenkamp & Barsalou, 2012 ; Hasenkamp et al., 2012 ; Jazaieri et al., 2014 ; Jha, Morrison, Parker, & Stanley, 2017 ; Morrison, Goolsarran, Rogers, & Jha, 2014 ; Mrazek, Franklin, Phillips, Baird, & Schooler, 2013 ; Stawarczyk & D’Argembeau, 2015 ; Vago & Zeidan, 2016 ; Zanesco et al., 2016 ), the explicit implications of long-term meditation practice on specific characteristics of self-generated thought and mind-wandering, such as the frequency, duration, content, and affect of spontaneous thoughts, with several exceptions ( Banks et al., 2015 ; Evans & Segerstrom, 2011 ; Jazaieri et al., 2016 ; Sanger & Dorjee, 2016 ) have remained relatively unexplored. This is notable given that many of the benefits that come from meditation practice may be due to improved attention regulation and enhanced meta-awareness of ongoing thought processes ( Lutz et al., 2008 ; Lutz et al., 2008 ; Menezes et al., 2013 ). Evidence for this comes from findings that focused attention meditation techniques have been shown to lead to an increased awareness of ongoing experience, emotions, and a decreased frequency with which mind-wandering occurs both during and outside of meditation practice ( Baird, Mrazek, Phillips, & Schooler, 2014 ; Brandmeyer & Delorme, 2018 ; Dorjee, 2016 ; Sanger & Dorjee, 2016 ; Sze, Gyurak, Yuan, & Levenson, 2010 ). Mind-wandering in the context of meditation provides individuals a unique and intimate opportunity to examine the nature of mind-wandering and cultivate awareness of ongoing thought dynamics through the cyclical nature of the meditative process ( Fig.2 ). Many meditation and mindfulness based practices emphasize the practice of non-judgmentally returning one’s attention to the breath or to the focal aim of the meditation practice ( Baer, 2015 ; Davidson, 2010 ; Kabat-Zinn, 2003 ; Vago & Zeidan, 2016 ; Zeidan, Johnson, Diamond, David, & Goolkasian, 2010 ). Through this training, meditation practitioners learn to develop sustained attentional focus ( Ainsworth, Eddershaw, Meron, Baldwin, & Garner, 2013 ; Lutz et al., 2008 , 2009 ; Tang & Posner, 2009 ), metacognitive awareness of thoughts, feelings, and emotions (Baird, Mrazek, et al., 2014; Brandmeyer & Delorme, 2018 ; Dorjee, 2016 ; Sze et al., 2010 ), while simultaneously cultivating equanimity towards the content of thoughts, judgments, and experience ( Hofmann et al., 2011 ; Jazaieri et al., 2014 ; Weng et al., 2013 ). It is important to emphasize that meditation practices are not intended to lead to a cessation of mind-wandering, but rather a mitigation of its potential deleterious effects and an improved awareness and openness to the passing nature of experience ( Desbordes et al., 2015 ).

The cycle of meditation and mind-wandering during a focused attention meditation practice. The neurocognitive model of how meditation cultivates awareness of mind-wandering by directly engaging the neural substrates implicated in attention regulation, perception, and meta-awareness. This cycle highlights the role of awareness of spontaneous thought and the cyclical observation and detection of involuntary shifts of attention as being at the core of focused meditation practice. Here a meditator begins meditating by 1) the focusing of attention, to then have their 2) attention shift to content of spontaneous thought, until the meditator 3) becomes aware the mind has been off focus, and 3) reorientes attention back to the focus of meditation.
The principal cycle of a focused attention meditation practice is to direct and maintain the focus of attention on a specified object (usually singular) of meditation. That is, one’s attention is completely occupied with focusing on the breath, a mental image, physical sensation, visual object, sound, or mantra ( Travis & Shear, 2010 ; Wahbeh, Sagher, Back, Pundhir, & Travis, 2018 ). In open monitoring meditation practices (OM), the meditator focuses on cultivating awareness and acceptance of what is occurring in the present moment without any pre-determined focal object ( Ainsworth et al., 2013 ; Colzato, Szapora, & Hommel, 2012a ; Kabat-Zinn, 2003 ; Travis & Shear, 2010 ). Additional practices that help to facilitate increased awareness of the occurrence and content of mind-wandering are often added such as labeling emotions, thoughts, and the sensations in the body. These techniques aim to help prevent the meditator from harshly judging themselves once they become aware that the mind has wandered, while providing practitioners with a structured framework for effectively working with their thoughts ( Tang et al., 2015 ).
Equanimity, which can be defined as an even-minded mental state or dispositional tendency toward all experiences or objects, regardless of their origin or their affective valence (pleasant, unpleasant, or neutral), is a key and central component of the meditation cycle and mindfulness, as with time, it transforms the way meditators respond and relate to their own internal thoughts and experience ( Davidson, 2010 ; Desbordes et al., 2015 ). These techniques are also thought to facilitate the reappraisal of thoughts as passing phenomenon, while simultaneously associating the direct experience of the physical sensations associated with thoughts with a newly cultivated response of equanimity ( Hölzel et al., 2011 ). Together, we suggest that these processes coalesce in creating a self-regulated closed-loop feedback system for the mind and body, eventually leading to enhanced detection of the early sensory signals associated with introspection and spontaneous thought processes during the meditation cycle ( Fox et al., 2012 ; Kerr et al., 2011 , 2013 ; Sze et al., 2010 ).
Evidence from our previous research implementing an experience sampling paradigm during an hour of focused meditation suggests that long-term meditation practice is associated with a reduced frequency of mind-wandering episodes ( Brandmeyer & Delorme 2018 ). We additionally found a greater correspondence between the self-reports of meditation depth and the simultaneously measured electroencephalography (EEG) activity in long-term meditation practitioners, as compared to intermediate and novice practitioners. While this study demonstrated a direct effect of accrued meditation experience and reduced mind-wandering during meditation, it does not decipher whether this is due to a heightened meta-awareness of when the minds wander (e.g., detecting and redirecting their attention back to the focus of meditation more frequently), or improvements in sustained attention (engaged in longer periods of meditative absorption), or both. Additional research investigating the effects of meditation practice on mind-wandering have also found significant reductions in the number of mind-wandering events following a brief mindful breathing exercise ( Mrazek et al., 2012 ), following one and three months of intensive meditation retreat practice ( Zanesco et al., 2016 ), as well as following several weeks of meditation practice ( Banks et al., 2015 ; Jazaieri et al., 2014 ; Jha et al., 2017 ; Morrison et al., 2014 ; Mrazek et al., 2013 ).
While further research should aim to garner a more granular understanding of these processes, we posit that this increased detection of mind-wandering observed in meditation practitioners most likely reflects a combination of enhanced attentional stability as well as improved meta-awareness, a position evidenced by a wealth of literature supporting the effects of both enhanced attention regulation ( Ainsworth et al., 2013 ; Lutz et al., 2008 ; Menezes et al., 2013 ; Tang & Posner, 2009 ; van den Hurk, Giommi, Gielen, Speckens, & Barendregt, 2010 ) and enhanced metacognitive awareness (Baird, Mrazek, et al., 2014; Fox & Christoff, 2014 ; Sanger & Dorjee, 2016 ) in meditation and mindfulness practitioners.
Variations in tradition and technique are also likely to influence the degree of interplay between the focusing of attention, the aperture of awareness, and the processes underlying the evaluation of thought content and mind-wandering ( Vago & Silbersweig, 2012 ; Vago & Zeidan, 2016 ). In the case of meditation practices in which the intentional generation of thoughts are explicitly employed - such as in loving kindness meditation (LKM) - these practices may require the individual to draw on ideas of the self and others, as well as past memories and conceptual notions of abstract concepts that often involve working with thought content explicitly and in ways that overlap with the processes involved in the generation of spontaneous thoughts. For example, in the case of focused attention, the target skill of focusing attention on the breath directly competes (opposing constructs) with mind-wandering for a limited set of cognitive resources. In the case of LKM, the thoughts specific to the practice become the spotlight of an internally directed self- or other-referential state of attention ( Petersen & Posner, 2012 ). Theoretically, it may be impossible for meditators to practice LKM and have a dual awareness of mind-wandering at the same time because of the cognitive difficulty involved in holding two separate trains of thought simultaneously (although successive thoughts on the order of 1 second may be possible; Delorme & Brandmeyer, 2019 ). This is supported by a recent meta-analysis showing that during FA and OM meditation practices, with the only exception being LKM, large meta-analytic clusters were present in the posterior dorsolateral prefrontal cortex, as well as in the premotor and supplementary motor cortices, all frontal areas that are heavily implicated in higher order cognitive functions such as conflict detection, sustained attention, and emotional regulation ( Amodio & Frith, 2006 ; Fox et al., 2016 ; Hanakawa et al., 2002 ).
A recent study by Fox and colleagues (2014) reported anatomical changes (e.g., increased cortical thickness; Lazar et al., 2005 ) in the frontopolar brain area across all three meditation practices ( Fox et al., 2016 , 2014 ). The frontopolar cortex is an area which has been functionally linked to meta-awareness and metacognitive capacity ( Baird, Smallwood, Gorgolewski, & Margulies, 2013 ; Fleming & Dolan, 2012 ; Fleming, Huijgen, & Dolan, 2012 ; Fleming, Weil, Nagy, Dolan, & Rees, 2010 ; Fox et al., 2015 ; McCaig, Dixon, Keramatian, Liu, & Christoff, 2011 ), as well as in the evaluation of self-generated information ( Christoff et al., 2003 ). As the frontopolar cortex serves as a hub for the frontoparietal control network (FPCN; ( Dixon et al., 2018 ; Ptak, 2012 ; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005 ; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2012 ; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008 ), it may also facilitate the alternation between endogenous and exogenous orientations of attention ( Brandmeyer, 2017 ; Burgess, Dumontheil, & Gilbert, 2007 ; Dixon et al., 2018 ; Ptak, 2012 ; Spreng et al., 2012 ). Thus, while meta-awareness and metacognitive capacity appear to be cognitive features of all the aforementioned forms of contemplative practice, focused attention meditation practice appears to be specifically implicated in higher order cognitive functions, generally involving some focal point to be returned to upon the detection of spontaneous thought processes. For purposes of clarity we focus on research findings from studies investigating the effects of focused attention meditation practices (unless noted), as they are some of the most widely scientifically studied forms of meditation practice and best suited for exploring how contemplative practices may influence this phenomenology and the interplay between these constructs.
The phenomenology of spontaneous thought and the influence of contemplative practice
First-person accounts reveal spontaneous thought to be an incredibly complex phenomenon involving a multitude of time domains, and intellectual and creative content ( Andrews-Hanna, Reidler, Huang, & Buckner, 2010 ; Fox & Christoff, 2014 ; Fox, Nijeboer, Solomonova, Domhoff, & Christoff, 2013 ; Klinger, 2009 , 2013 ; McMillan, Kaufman, & Singer, 2013 ; Seli et al., 2018 ; J. Smallwood, 2013 ). It has been shown that mind-wandering episodes typically involve thinking about oneself, others, remembering the past, and planning for the future ( Buckner, Andrews-Hanna, & Schacter, 2008 ; Gusnard, Raichle, & Raichle, 2001 ; Raichle et al., 2001 ). Across methodologies used to investigate self-generated forms of thought, research in healthy non-clinical populations shows that they are rated as mildly pleasant, positive, and enjoyable, and on average display a mild positive affect bias ( Fox & Christoff, 2018 ). Research also suggests that mind-wandering serves as a mnemonic process, involving a variety of episodic forms of autobiographical memory facilitating an «autonoetic consciousness» unique to the human capacity of the awareness of self ( Tulving & Craik, 2005 ; Vago & Zeidan, 2016 ). Tulving (2005) suggests that it is this autonoetic capacity which provides us the fundamental framework for the advancements in technology, society, our intelligence, and the intrinsic abilities necessary to navigate our complex internal and external environment. The ability to mentally move through time has been referred to as ‘mental time travel’ and has been directly linked to the episodic memory processes thought to generate mental content ( Tulving, 2002 ).
Prospective bias, positive affect, goal-directed planning and creativity
Behavioral studies reveal dynamic interactions between spontaneous thought and executive function, that appear to depend on the difficulty of task the person might be doing ( Smallwood, 2013 ; Smallwood & Andrews-Hanna, 2013 ). These findings support the context regulation hypothesis proposed by Smallwood and Andrews-Hanna (2013) which claims that the costs and benefits of mind-wandering partly depend on an individual’s ability to constrain task unrelated thought in situations that demand their attention ( Andrews-Hanna, Smallwood, & Spreng, 2014 ; Smallwood & Andrews-Hanna, 2013 ). In healthy participants when demands on an individual are particularly low, evidence suggests that attentional resources are redirected towards supporting spontaneous thought processes that are predominately prospective in nature ( Baird, Smallwood, & Schooler, 2011 ; D’Argembeau, Renaud, & Linden, 2011 ; J. Smallwood et al., 2011 ; Stawarczyk & D’Argembeau, 2015 ). In line with these findings, the prospective orientation of mind-wandering has been directly linked with the ‘current concerns’ of an individual ( Andrews-Hanna et al., 2014 ; Klinger, 2013 ; McVay & Kane, 2010 ) suggesting that mind-wandering may facilitate autobiographical planning and the planning of future goals ( Addis, Knapp, Roberts, & Schacter, 2012 ; Andrews-Hanna, Saxe, & Yarkoni, 2014 ; Baird et al., 2011 ).
These findings are supported by additional research showing that self-oriented thought increases the frequency of future thinking, and that prospective experiences mediate the memory advantage for self-referential information ( Klein, Robertson, Delton, & Lax, 2012 ). Baird and colleagues (2011) implemented a choice reaction task to explore the temporal focus (i.e., past-, present-, or future-oriented) and cognitive orientation (i.e., self-related or goal-directed) of participants’ thoughts and found that participants’ thoughts were predominately future-oriented and self-related. They also found that when thoughts were both self-related and goal-directed they were more frequently future-focused than present or past-focused. These findings provide strong evidence that mind-wandering facilitates goal-directed planning in relation to personal concerns. Furthermore, individuals with improved executive control have been shown to limit their self-generated thought to nondemanding or unimportant contexts ( Barron, Riby, Greer, & Smallwood, 2011 ). In line with these findings, social thoughts pertaining to one’s future have been shown to lead to subsequent positive thoughts, whereas social thoughts pertaining to one’s past precede negative mental content ( Ruby, Smallwood, Engen, & Singer, 2013 ).
Retrospective bias, negative affect, rumination and clinical conditions
Research has shown that mind-wandering focused on the past is directly associated with increased negative affect in laboratory conditions ( Ruby, Smallwood, Engen, & Singer, 2013 ; Smallwood et al., 2011 ; Stawarczyk, Majerus, & D’Argembeau, 2013 ) and in daily life ( Poerio, Totterdell, & Miles, 2013 ). In a seminal study using experience sampling implemented with a mobile phone application, Killingsworth and Gilbert (2010) collected data on 2,250 participants and found that mind-wandering was associated with a reduced sense of well-being when mind-wandering events focused on the past. Psychopathological states such as anxiety and depression have also been linked to self-generated experiences that have past-oriented and perseverative features ( Ottaviani & Couyoumdjian, 2013 ; Ottaviani, Shapiro, & Couyoumdjian, 2013 ), while an increased frequency of unaware mind-wandering was also associated with higher levels of depression ( Deng, Li, & Tang, 2014 ). A majority of neurocognitive disorders can be characterized by dysfunctional regulation of both context and content, with alterations in both processes likely to yield devastating consequences on cognitive functioning and well-being ( Andrews-Hanna et al., 2014 ). Furthermore, the neural mechanisms underlying spontaneous thought processes appear to play a direct role in clinical conditions such as post-traumatic stress disorder, depressive rumination, and a host of other mental health disorders where individuals have difficulty regulating the frequency of self-generated spontaneous thoughts ( Andrews-Hanna, Saxe, & Yarkoni, 2014 ; Berman et al., 2011 ; Ehlers, Hackmann, & Michael, 2004 ; Nolen-Hoeksema, 2000 ; Poerio, Totterdell, & Miles, 2013 ; Smallwood, Fishman, & Schooler, 2007 ; Smallwood, McSpadden, & Schooler, 2007 ; Whitfield-Gabrieli & Ford, 2012 ).
These deficiencies often manifest as increased distractibility or elevated levels of mind-wandering ( Smallwood, Fishman, et al., 2007 ; Watkins, 2008 ). Depressed individuals demonstrate an increased disposition for rumination and experience difficulties updating the contents of working memory and switching tasks ( Deng et al., 2014 ). This leads to preexisting goal states exerting a stronger influence on ongoing mental processes and thought content in depressed individuals than for normal individuals ( Andrews-Hanna et al., 2014 ; Fox et al., 2013 , 2015 ). Individuals who score higher on constructs related to depression and trait negative affect on questionnaires also rate their self-generated thoughts as more negative in valence ( Andrews-Hanna et al., 2013 ). Together these studies suggest that in the case of clinical and psychopathological conditions, an inability to monitor and disengage from the occurrence of excessive or distracting self-generated thoughts in a context-dependent manner is associated with impairments in wellbeing ( Andrews-Hanna, Smallwood, & Spreng, 2014 ).
Opposing constructs?
When compared to mindfulness which aims to facilitate attentional focus, clarity, and stability, spontaneous thought and mind-wandering are characteristically defined as the disruption of focused attention or task focus ( Smallwood & Schooler, 2006 ). Mind-wandering (spontaneous thought) has been shown to be particularly recurrent during nondemanding tasks and restful waking states ( Kane et al., 2007 ; Singer, 1966 ), and appears to have a distinctly ‘mindless’ quality ( Smallwood & Schooler, 2006 ), such as rapid and automatic responding during continuous performance tasks ( Smallwood, Baracaia, Lowe, & Obonsawin, 2003 ), eye-movements during reading that demonstrate a reduced processing of the lexical or linguistic properties of what is being read (indicating perceptual decoupling; Reichle, Reineberg, & Schooler, 2010 ), as well as absent-minded forgetting ( Smallwood et al., 2003 ; Smallwood & Schooler, 2006 ). The degree to which the constructs of mindfulness and mind-wandering oppose one another was explored in a set of studies by Mrazek and colleagues (2012) wherein they addressed the relationship between a dispositional measure of mindfulness using the Mindful Attention and Awareness Scale (MAAS; Brown & Ryan, 2003 ) and converging measures of both self-reported and indirect markers of mind-wandering. Negative correlations between dispositional mindfulness and four measures of mind-wandering suggest a relatively opposing relationship between the two constructs. They additionally found that eight minutes of mindful breathing reduces behavioral indicators of mind-wandering during a Sustained Attention to Response Task compared with both passive relaxation and reading. While these findings suggest a relatively opposing nature of the two constructs ( Mrazek et al., 2012 ), the degree to which thoughts are constrained (or unconstrained) based on the task, or on the objectives of a given meditation practice is likely to influence the degree to with which these constructs oppose one another.
This is supported by findings from Colzato and colleagues (2012b) who found that open monitoring meditation (which promotes the ability to observe ongoing mental content and attend to various transient stimuli) increased creativity in an idea generation task, whereas focused attention meditation (training the ability to focus attention and awareness) did not. In an additional set of studies by Ostafin and Kassman (2012) , the authors showed that a greater tendency toward mindfulness (which is generally taught as a style of open monitoring meditation), as assessed through the MAAS, was associated with an increased chance of solving the puzzles, and that a brief session of mindfulness meditation improved both situational mindfulness and problem solving. The authors take the position that mindfulness and the emphasis placed on the ‘present moment’ experience reduces the tendency toward habitual responses when searching for the solution to a creative problem. Zedelius and colleagues (2015) conducted a study to explore whether creative problems approached through an analytic meditation strategy or through an insight meditation (i.e., sudden awareness of a solution) would impact measures of creativity. They found impaired problem solving when approaching problems with insight meditation, whereas increased problem solving performance was associated with the use of the analytic (i.e., thought-based) meditation approach.
Baird and colleagues (2012) explored the hypothesis that mind-wandering would be associated with enhanced creativity through the use of incubation tasks that systematically varied in their levels of attentional demand and thus in their conduciveness to mind-wandering. Their findings suggest that performing an undemanding task during the incubation period improved creative performance to a greater extent than performing a demanding task, resting, or taking no break. They additionally found that scores on the daydreaming frequency subscale of the Imaginal Processes Inventory - a questionnaire measure that assesses individual’s tendency for mind-wandering in everyday life ( Gold and Gold, 1982 ), correlated positively with scores on a classic creativity task both for repeated exposure and new exposure problems on the task. This last result suggests that specific types of spontaneous, unrelated thoughts facilitate creative problem solving, and that individuals who mind-wander more frequently in their daily lives may also be more creative in general. Although mind-wandering may be linked to compromised performance on a variety of experimenter-defined tasks ( Barron et al., 2011 ; McVay & Kane, 2010 ), it also serves to facilitate creative ideas ( Baird et al., 2012 ; Schooler et al., 2014 ) and memory consolidation ( Addis et al., 2012 ).
Spontaneous thought content, affect, and well-being
Well-being is related to a complex variety of factors including culture, socioeconomic status, health, the quality of interpersonal relations, and specific psychological processes (Dinero, Conger, Shaver, Widaman, & Larsen-Rife, 2008). Research findings suggest that both the content of self-generated thought and the context under which it occurs significantly influence both the cognitive and affective impact of the experience, elucidating why the experience of mind-wandering can be detrimental for some individuals yet beneficial for others ( Andrews-Hanna et al., 2014 ; Andrews-Hanna, Smallwood, & Spreng, 2014 ). Clinical research suggests that the ability to distance oneself and observe the ongoing internal train of thoughts plays a vital role in psychological wellbeing ( Farb et al., 2007 ). Within the domain of cognitive psychology, latent conceptions of self are thought to underlie (to a significant degree) our thoughts and emotions and directly impact brain function ( Hofmann, Schmeichel, & Baddeley, 2012 ). It has been proposed that one of the primary mechanisms by which contemplative practices affect well-being is by targeting and altering maladaptive self-referential thought patterns ( Dahl et al., 2015 ). The content regulation hypothesis ( Andrews-Hanna et al., 2014 ) suggests that a direct interaction between mood and content influence the relationship to affect ( Christoff, Gordon, Smallwood, Smith, & Schooler, 2009 ; Deng et al., 2014 ; Fox et al., 2018 ; Poerio et al., 2013 ; Ruby et al., 2013 ; Smallwood et al., 2011 ), with research directly linking the retrospective orientation of mind-wandering to premature aging ( Epel et al., 2013 ) and negative affect.
Trait and state effects, emotion reappraisal, and the role of acceptance
Meditation training is thought to induce both state and trait effects. The term state effects refers to the distinction between ordinary mental states, and the specific mental states that are experienced during various styles of meditation practice. Changes that occur over months or years are referred to as trait effects. It is generally accepted that state effects translate to long-term trait effects with practice ( Cahn & Polich, 2006 ). For example, thought reappraisal during meditation can be considered a state effect that occurs during the practice of meditation, however long-term training may lead to thought reappraisal that extends beyond formal meditation practice evolving into what could be considered a trait. Accumulative exposure to and awareness of the ongoing cognitive and affective dynamics involved in spontaneous thought processes plays a central role in the mechanisms underlying meditation practice ( Hasenkamp et al., 2012 ), and the benefits observed in the context of emotion reappraisal ( Hölzel et al., 2011 ). A growing body of literature now suggests that the key factors implicated in the regulation of emotion involve reappraisal, exposure, extinction, and reconsolidation ( Hölzel et al., 2011 ). Hanley and Garland (2014) found that mindfulness practice led to an increase in positive reappraisal of thoughts. They argue that mindfulness practice facilitates positive reappraisal, with reappraisal functioning as an adaptive process through which stressful events are reconstructed as beneficial, meaningful, or benign.
Additional literature suggests that during mindfulness meditation meditators allow themselves to be affected by the experience of their thoughts while refraining from engaging in internal reactivity towards them by cultivating acceptance of bodily and affective responses ( Hart, 2011 ; Hölzel et al., 2011 ). Thus, the accumulative impact of meditation training on the reappraisal of ongoing thoughts that occur during mind-wandering episodes (or even during more constrained forms of self-generated thought) may help to facilitate enhanced awareness and reduced reactivity to the content of spontaneous thoughts, and this may serve as one of the key mechanisms by which meditation practices shape the cognitive-affective lens through which we perceive the content of mind-wandering. Research investigating the effects of a brief attention-monitoring combined with an acceptance mindfulness training program found significantly reduced mind-wandering when compared with an attention-monitoring only mindfulness training program ( Rahl, Lindsay, Pacilio, Brown, & Creswell, 2017 ). These findings directly support the key role of acceptance implicated in mind-wandering, and further elucidate the cognitive mechanisms underlying mindfulness training ( Chiesa & Malinowski, 2011 ; Creswell & Lindsay, 2014 ; Franklin et al., 2013 ; Moore & Malinowski, 2009 ; Teper & Inzlicht, 2013 ). This research suggests that the aspect of ‘acceptance of present-moment experience’ cultivated in mindfulness training, which includes the acceptance of spontaneous thought and mind-wandering, leads to reduced emotional reactivity to adverse thought content allowing for an improved capacity for the reallocation of attention. These findings are supported by neuroimaging research which has shown notable similarities in the brain regions influenced by mindfulness meditation and those involved in mediating fear extinction, namely the hippocampus, amygdala, medial PFC, and the ventromedial PFC ( Goldin & Gross, 2010 ; Hölzel et al., 2011 ; Lazar et al., 2000 ; Lou et al., 1999 ; Luders, Toga, Lepore, & Gaser, 2009 ; Newberg et al., 2001 ; Unsworth & McMillan, 2013 ). Furthermore, these regions (with the exception of the amygdala) coincide with the key hubs in the broader neural substrates of the default network (DMN), and as we will discuss in the following section, are heavily implicated during self-generated thought and mind-wandering.
Meditation, affect, and emotion regulation
Several recent studies have explored how the constructs of mindfulness and the valence of self-generated thought may interact. While these studies implement measures of mindfulness assessed via questionnaire (which are notoriously biased and have been the source of significant criticism; Van Dam et al., 2018 ), several recent studies provide evidence for the direct relationship between mindfulness and the valence of self-generated thought. Evans and colleagues (2011), as well as Andrews-Hannah and colleagues (2013), correlated participants scores on the Five Facets Mindfulness Questionnaire (FFMQ), which purports to measure trait levels of mindfulness ( Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006 ), alongside the affective qualities of self-generated thoughts. They both found that higher trait mindfulness scores significantly predicted more emotionally positive thought content. In support of these findings, Frewen and colleagues (2008) found that dispositional mindfulness as measured by their Mindful Attention Awareness Scale (MAAS; Brown & Ryan, 2003 ) negatively correlated with the frequency of negative thoughts. These findings correspond with research suggesting that mindful individuals report heightened positive affect in general ( Brown & Ryan, 2003 ; Hölzel et al., 2011 ; Jazaieri et al., 2014 ; Teper et al., 2013 ) and that meditation may facilitate positive affect. This is supported by findings by Jazaieri and colleauges (2016) who found that the number of hours of seated compassion meditation practice during nine weeks of compassion meditation training predicted the affective qualities of off-task thought. They found that the number of hours of practice predicted reduced off-task thoughts to both negative and neutral topics, and increased off-task thoughts to positive topics. Research studying the neural mechanisms underlying the regulation of emotion have been directly linked to brain regions associated with attention and cognitive control, including the dorsomedial, dorsolateral, and ventrolateral prefrontal cortex, as well as the posterior parietal cortex ( Ochsner & Gross, 2005 ; Ochsner et al., 2004 ). Meditation and mindfulness practices may mediate emotion regulation and emotional reactivity to self-generated thought content by strengthening prefrontal cognitive control mechanisms via suppression of activity in the amygdala. Supporting this hypothesis, diminished activations in the amygdala in response to emotional stimuli have been found in experienced meditation practitioners ( Tang et al., 2015 ).
Spontaneous thought is mediated by brain networks implicated in meditation
Significant progress over the last decade has been made in identifying the brain networks underlying mind-wandering and the generation and maintenance of self-referential thought processes. Research consistently shows default mode network (DMN) activations during both probe-caught and self-reported episodes of mind-wandering ( Smallwood & Schooler, 2015 ), in addition to increased activation when individuals are at rest in an MRI scanner ( Raichle et al., 2001 ). Interestingly, the emphasis on the flexible monitoring of self-referential thought processes and ongoing experience during meditation has been theoretically attributed to the increased functional connectivity within the DMN ( Brewer et al., 2011 ; Garrison, Zeffiro, Scheinost, Constable, & Brewer, 2015 ; Jang et al., 2011 ; Tang et al., 2015 ; Wells et al., 2013 ), and between the DMN and the dorsolateral prefrontal cortex (dlPFC; Brewer et al., 2011 ) that is observed in experienced meditation practitioners. It is of importance to note here that the lateral PFC is a region associated with both exogenous and endogenous attentional states, meta-awareness, and executive functioning ( Froeliger et al., 2012a ; Hasenkamp & Barsalou, 2012 ; Teper et al., 2013 ).
Research in functional magnetic resonance imaging (fMRI) has identified the regions that comprise the DMN ( Fig. 3 ; Raichle et al., 2001 ; Raichle, 2015 ) including the medial prefrontal cortex (specifically, the dorsal medial prefrontal cortex (dmPFC), the rostral anterior cingulate, and parts of the anterior and ventral mPFC), the lateral frontal cortex (the superior frontal cortex and the inferior frontal gyrus), the medial parietal cortex (the posterior cingulate and retrosplenial cortex), the medial temporal lobe (the hippocampus and parahippocampal cortices), the lateral parietal cortex (spanning the angular gyrus and the posterior supramarginal gyrus/TPJ), and the lateral temporal cortex (extending anteriorly to the temporal poles). The DMN also includes large areas of the cerebellum (including Crus I and Crus II subdivisions) and the striatum (the medial wall of the caudate and the posterior putamen; ( Andrews-Hanna et al., 2010 ). While a set key regions originally identified through the repeated observation of patterns of deactivation during goal-directed tasks (as compared to passive control conditions) were thought to reflect the DMN ( Raichle et al., 2001 ), recent findings have shown that during goal-directed tasks of an internal nature, significant task-dependent variability in DMN activations have been observed ( Andrews-Hanna et al., 2010 , 2014 ; Andrews-Hanna et al., 2014 ).

Template maps for the cortical networks implicated in the cognitive processes underlying the meditative cycle: default network (DN), frontoparietal control network (FPCN), salience network (SN), and dorsal attention network (DAN). Color intensities indicate factor loading of each voxel with the network template in the reference dataset. Used with permission from Shaw et al., (2015) .
Recent advances in resting-state functional connectivity (rsFC) analysis have now enabled a more comprehensive understanding of the functionally integrated relationship between spatially separated brain regions and the complexity of the DMN, revealing its distinct yet interacting subsystems ( Andrews-Hanna et al., 2010 , 2014 ; Fox et al., 2015 ; Gusnard et al., 2001 ). Andrews-Hanna and colleagues recently identified three key subsystems that constitute the DMN. A medial temporal subsystem comprised of the hippocampus, the parahippocampal cortex, the retrosplenial cortex (RSC), the posterior inferior parietal lobe, and the ventromedial prefrontal cortex (vmPFC). This subsystem significantly corresponds with meta-analytic maps pertaining to past and future autobiographical thought (i.e., autobiographical, past, future), episodic memory (i.e., episodic, memories, remember, recollection, recall) and contextual retrieval ( Andrews-Hanna et al., 2014 ). A dorsal medial subsystem comprises the dorsal medial PFC (dmPFC), the temporoparietal junction (TPJ), the lateral temporal cortex, and the temporal pole, regions that correspond with meta-analytic maps pertaining to social cognition (i.e., mentalizing, social, person, theory of mind, mental, scenarios), as well as story comprehension and semantic and conceptual processing (i.e., sentence, story, meaning, knowledge, language, word, syntactic). A third subsystem is comprised of regions along the cortical midline, including the anterior medial PFC (amPFC) and the posterior cingulate cortex (PCC) exhibit strong functional coherence with both subsystems and are hypothesized to act as functional hubs, allowing information to transfer between subsystems. The PCC, angular gyrus, and amPFC are the most consistently engaged regions within the DMN, regions directly associated with self-related processes (i.e., self-referential, self, autobiographical, personal), emotion/evaluation (i.e., positive, negative, moral), and social and mnemonic processes, features shared by both the dorsal medial and medial temporal subsystem (i.e., social, person, mentalizing, recollection, retrieval, memories; Andrews-Hanna, Saxe, & Yarkoni, 2014 ). Additional findings suggest that the PCC hub is a heterogeneous brain structure, with subdivisions characterized by distinct patterns of structural and functional connectivity that echo the neural signals from several additional large-scale brain networks. These observations suggest that the broader PCC can be viewed as an important integration zone supporting bottom-up mechanisms of attention that enable behaviorally relevant sources of information to be drawn from memory and perceptual information. A recent paper by Andrews-Hanna and colleagues (2014) argues that together these neural systems support a majority of the mental content underlying self-generated thought.
Functional connectivity in regions of the DMN, a measure of the temporal correlation of the BOLD signal between these regions, has also been found to differ between meditators and controls, not only during meditation but also at rest ( Brewer et al., 2011 ; Pagnoni, 2012 ; Taylor et al., 2013 ). This suggests that meditation training may alter the behavioral state that individuals enter when given the standard resting-state instructions. One interpretation of these findings would be that increased functional connectivity in the DMN may in turn reduce blood-oxygen-level-dependent imaging (BOLD) activity, reflecting an increased efficiency in the distribution of cognitive resources ( Baars, 2005 ). Another possible explanation for the relationship between reduced BOLD activity and increased functional connectivity in advanced practitioners may be due to the increased connectivity between networks involved in monitoring and attention that overlap with the DMN, rather than specifically DMN. An additional alternative explanation may be that meditators engage in less spontaneous thought during rest and therefore show reduced BOLD signal in the DMN (although this does not account for the increased functional connectivity).
However, even if it were the case that the increased functional connectivity in the DMN reflects connections to regions overlapping with other networks, together these findings support the notion that both endogenous and exogenous forms of cognitive activity such as mind-wandering and meditation both recruit regions in both the DMN in addition to overlapping regions and networks implicated in the regulation of executive functions. While the relationship between the DMN and mind-wandering has been extensively studied within neuroscience ( Dixon, Fox, & Christoff, 2014 ; Kucyi, Salomons, & Davis, 2013 ), activity in the DMN alone does not capture the broader neural landscape associated with the occurrence, duration, frequency, and maintenance of mind-wandering and spontaneous thought ( Anticevic et al., 2012 ; Berman et al., 2011 ; Stawarczyk, Majerus, Maquet, & D’Argembeau, 2011 ; Vanhaudenhuyse et al., 2011 ; Whitfield-Gabrieli & Ford, 2012 ). A general picture of brain activity involved in mind-wandering and spontaneous thought is beginning to emerge, implicating brain networks beyond the DMN such as the frontoparietal control network (FPCN), the dorsal attention network (DAN), and the salience network (SN; Christoff et al., 2009 ; Dixon et al., 2018 ; Ellamil et al., 2016 ; Fox et al., 2016 ). The FPCN includes lateral prefrontal cortex, precuneus (PCu), the anterior extent of the inferior parietal lobule (aIPL), medial superior prefrontal cortex (msPFC) and the anterior insula (aINS; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2012 ). This network has been proposed to modulate top-down mechanisms involved in sustaining both endogenous and exogenous forms of attention allocation ( Spreng et al., 2012 ). As discussed in Smallwood et al (2012) the ability to generate and sustain an internal train of thought is facilitated through cooperation between autobiographical information provided by the DMN and a frontal–parietal control network which helps sustain and buffer internal trains of thought against disruption by the external world. Spreng and colleagues (2012) suggest that the FPCN facilitates goal-directed cognition, which functions as a gatekeeping system by moderating the dynamic balance between activations in the DMN and the DAN ( Fig. 3 ). It may also facilitate alternating or competing goal representations while maintaining directed attention to a given external task (i.e. driving, running; Spreng et al., 2012 ). Concurrent activations in both the DMN and core regions of the executive functioning network (dorsolateral PFC, mPFC, ACC), networks that were traditionally considered independent, anti-correlated, and thought to compete for cognitive resources, have been shown to co-activate during mind-wandering episodes, increasingly so when subjects reported being unaware of mind-wandering ( Christoff et al., 2009 ; Fox et al., 2015 ).
Similar to the work of Andrews-Hanna and colleagues that fractionated the DMN, recent research by Dixon and colleagues (2018) examined patterns of FPCN functional connectivity across multiple conditions of varying cognitive demand, identifying two distinct subsystems within the FPCN. These two FPCN subsystems exhibited unique patterns of functional connectivity with the DMN and the DAN. The first subsystem FPCN(a) includes the rostrolateral prefrontal cortex, anterior inferior parietal lobule, presupplementary motor area, and middle temporal gyrus, and exhibited significantly stronger measures of connectivity with the DMN than the DAN. The FPCN(b) consists of the intraparietal sulcus, posterior inferior frontal sulcus/inferior frontal junction (IFS/IFJ), posterior superior frontal sulcus, and left posterior middle temporal gyrus, and exhibited the opposite pattern. These findings provide new evidence suggesting that the organization of the FPCN is both flexible and heterogeneous, and that it may emerge from separable DMN and DAN processing streams ( Dixon et al., 2014 , 2018 ). The authors propose that FPCN(a) may be preferentially involved in the regulation of introspective processes, whereas FPCN(b) may be preferentially involved in the regulation of visuospatial perceptual attention.
A number of additional structural and functional MRI studies on mindfulness training have investigated the neuroplasticity in brain regions supporting the regulation of attention. The anterior cingulate cortex (ACC), another key hub of the FPCN, is an area in the brain that has been consistently linked to improvements in attention regulation following training in mindfulness ( Hölzel et al., 2011 ; Tang & Posner, 2009 , 2014 ; Tang, Rothbart, & Posner, 2012 ). The ACC and the fronto-insular cortex are thought to enable executive attention and control by detecting the presence of conflicts emerging from incompatible streams of information processing, thus facilitating cognitive processing through long-range connections to other brain areas ( van Veen & Carter, 2002 ). These mechanisms may work synergistically by establishing a process of enhanced meta-awareness and self-regulation following long-term meditation practice (Baird, Mrazek, et al., 2014; Baird et al., 2013 , 2013 ; Dorjee, 2016 ; Fleming & Dolan, 2012 ; Sanger & Dorjee, 2016 ; Tang et al., 2015 ). Furthermore, the perigenual ACC is implicated in emotional feeling states, evidenced by patterns of activation that correspond with the valence of stimuli ( Roy, Shohamy, & Wager, 2012 ). However, its role appears to be specifically related to the process of attributing conceptual meaning to bodily sensations and interweaving feeling states with self-referential thinking in addition to the capacity to identify and understand interoceptive feeling states ( Fleming et al., 2010 ; Fox et al., 2018 ; Roy et al., 2012 ).
According to Hasenkamp and colleagues’ (2012) study, when attention was reoriented back to a focused attention meditation after an episode of mind-wandering, increased activations in the lateral PFC and inferior parietal cortex were observed, suggesting that executive resources were recruited to deactivate the DMN by decoupling the node shared by the FPCN and the DMN. Furthermore, increased activity in the dorsolateral PFC (dlPFC), a central hub of the FPCN that has been repeatedly implicated in studies of focused attention and executive control, was observed during focused attention meditation ( Brewer et al., 2011 ). The work of Hasenkamp and Barslow (2012) investigated the brain networks directly implicated in the various phases of the meditation cycle. These findings highlighted the activity associated with the transitions between mind-wandering and a return to focused-attention, highlighting the key role of the salience network in signaling the detection of mind-wandering and the relaying of this information to the executive network. Metacognitive awareness of one’s thoughts, along with attention and performance, have also been directly linked the rostrolateral PFC (rlPFC; Fleming & Dolan, 2012 ; McCaig et al., 2011 ). Research investigating the neural correlates of lucid dreaming, wherein individuals become aware of and are engaged in the progression and content of their dreams, show that individuals who report a high degree of lucidity show increased grey matter volume in the medial and rlPFC, as well as enhanced rlPFC activity during tasks that require the subjects to monitor the contents of their thoughts ( Filevich, Dresler, Brick, & Kühn, 2015 ).The FPCN has also been widely implicated in lucid rapid eye movement (REM) sleep when compared to non-lucid REM sleep ( Dresler et al., 2012 ). Thus, accumulating research suggests that the FPCN plays a direct role in the control processes related to the meta-awareness of spontaneous thought and mind-wandering by monitoring and constraining the transitions between mental states by suppressing and directing the degree spontaneity ( Fox & Christoff, 2014 ). Meditation practice may target these monitoring mechanisms through the active process of detecting mind-wandering and the redirecting of attention.
Neuroimaging results show that mindfulness meditation practitioners also exhibit significantly greater rsFC in the DAN when compared with meditation naive individuals, and that mindfulness meditation practice in the MRI scanner (msFC) was associated with increased functional connectivity when compared to resting state levels (i.e., msFC > rsFC) between the DAN and DMN and the right PFC node of the salience network ( Froeliger et al., 2012a ). These findings suggest that mindfulness practice enhances functional connectivity within attentional networks as well as across broadly distributed brain regions sub-serving the regulation of introspective, attentional, self-referential, and emotional processes ( Brewer et al., 2011 ; Dixon et al., 2014 ; Froeliger et al., 2012b ; Garrison et al., 2014 ). Additional neuroimaging evidence also indicates that sustained activity in the salience network has been observed during meditation in long-term practitioners ( Fig. 3 ; Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007 ; Doll, Hölzel, Boucard, Wohlschläger, & Sorg, 2015 ; Hasenkamp et al., 2012 ). The salience network includes the bilateral anterior insula, lateral orbitofrontal cortex (OFC), anterior and mid-cingulate cortex, amygdala, and hypothalamus and is thought to facilitate the identification of relevant and salient stimuli and sustained cognitive focus ( Doll et al., 2015 ; Menon & Uddin, 2010 ; Mooneyham & Schooler, 2013 ; Ptak, 2012 ; Seeley et al., 2007 ). Biases in affective and perceptual attention can be thought to reflect natural constraints, which serve to capture and sustain attention on a focal source ( Christoff et al., 2016 , 2016 ; Irving, 2016 ; Todd, Cunningham, Anderson, & Thompson, 2012 ). Although evidence for a relationship between mind-wandering and nature of these constraints is limited, recent studies on depression and anxiety suggests that the brain’s salience network may play a key role ( McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014 ; Seeley et al., 2007 ; Young et al., 2017 ).
The salience network and activity in the dACC/pre-somatosensory motor area (pre-SMA) is thought to reflect the detection of conflict (e.g., mind-wandering when it occurs during an ongoing task), and may therefore be involved in determining the expected cost/benefit ratio tradeoffs of being either on- versus off-task. The insula has also been heavily implicated in the generation of self-generated thought and may be more heavily involved in viscero-somatic sensations (cardiac, respiratory, etc.) and feeling states (Fox 2018). The insula is also thought to play a role in detecting and signaling affective salience ( Markovic, Anderson, & Todd, 2014 ; Menon & Uddin, 2010 ; Seeley et al., 2007 ), with the anterior insula having been shown to be directly related to the degree to which thoughts trigger, or are triggered by, physiological arousal and other concrete bodily feelings ( Craig, 2002 ; Fox et al., 2018 ). Consistent with this hypothesis, awareness of visceral and internal psychological states, including heart rate and respiration is often referred to as interoception and has been consistently linked to activity in the insula (Craig and Craig, 2009; Critchley et al., 2004) in addition to metacognitive awareness ( Fleming and Dolan, 2012 ) and emotional self-awareness (Craig, 2004). Additional research has found that viscero-somatic information is progressively refined from the posterior to anterior insula, the anterior insula contributing directly to interoceptive awareness ( Craig, 2002 ). When comparing meditators during meditation versus non-meditation states, we find brain areas focused on self-regulation, focused problem-solving, adaptive behavior, interoception, monitoring body states, reorienting attention, and processing self-relevant information (Boccia et al., 2015). Given that approximately two-thirds of self-generated thoughts are emotional in nature, it is highly probable that self-generated thoughts regularly recruit brain areas and networks implicated in emotional processing ( Andrews-Hanna et al., 2013 ; Fox et al., 2018 ; Ruby et al., 2013 )
Meditation and modulations of attention regulation and sensory perception
Accumulating findings from contemplative neuroscience research suggest that meditation practice strengthens the top-down feedback mechanisms involved in the regulation of attention ( Brefczynski-Lewis et al., 2007 ; Chan & Woollacott, 2007 ; Jha, Krompinger, & Baime, 2007 ; Lutz et al., 2009 ; MacLean et al., 2010 ; Moore & Malinowski, 2009 ; Slagter, Davidson, & Lutz, 2011 ; Slagter, Lutz, Greischar, Nieuwenhuis, & Davidson, 2008 ; Valentine & Sweet, 1999 ; van den Hurk et al., 2010 ). According to the neurocognitive model developed by Posner and Petersen, attention can be divided into three different anatomically and functionally distinct networks. These networks implement the functions of alerting (i.e., the anticipatory preparation for an incoming stimulus), orienting (i.e., the directing of attention to a specific stimulus), and conflict monitoring (i.e., executive attention: resolving conflict between competing neural activity; Petersen & Posner, 2012 ; Posner & Petersen, 1990 ). Additional distinctions between different forms of attention involve combinations of these three components ( Posner & Rothbart, 2007 ). For example, sustained attention refers to the sense of vigilance during long continuous tasks and may involve both tonic alerting (i.e., intrinsic arousal that fluctuates on the order of minutes to hours) and orienting, whereas selective attention may involve either orienting (when a stimulus is present) and executive functions (when the processing of stored information is involved; Desimone & Duncan, 1995 ). Furthermore, regions of the dorsolateral prefrontal cortex that are heavily implicated in attentional processes that engage the executive network ( Curtis & D’Esposito, 2003 ; D’Esposito, 2007 ; Miller & Cohen, 2001 ) have been directly implicated in the practice of focused attention meditation ( Hofmann et al., 2012 ; Teper & Inzlicht, 2013 ).
These findings are consistent with the notion that meditation practices engage brain areas involved in inhibition ( Brefczynski-Lewis et al., 2007 ), as well as in the detection of conflict between goal states (i.e., focused attention on the breath conflicting with the occurrence of spontaneous thought; Hasenkamp & Barsalou, 2012 ; Hasenkamp et al., 2012 ). This is further evidenced by research by Moore and Malinowski (2009) and Chan and Woollacott (2007) who found reduced effects of distracting and conflicting information in the Stroop task in mindfulness meditators. Van den Hurk and colleagues (2010) found reduced interference by distracting flankers in the attention network test in mindfulness meditators, validating previous findings that mindfulness meditation leads to an increased flexibility in the orientation of attention by reducing the time needed to shift attention from one location to another ( Hodgins & Adair, 2010 ; Jha et al., 2007 ; van den Hurk et al., 2010 ). In a study comparing relaxation, open monitoring meditation, and focused attention meditation on performance on an emotional variant of the Attention Network Test (ANT), only focused attention and open monitoring practices were found to improve executive attention ( Ainsworth et al., 2013 ). Together these findings contribute to a large and accumulating body of evidence that mindfulness meditation targets mechanisms implicated in executive attention and the detection of conflicting mental states.
Electroencephalography (EEG) findings from our previous work ( Brandmeyer & Delorme, 2018 ) indicate that increased mid-frontal theta (4–6 Hz) and somatosensory alpha (8–12 Hz), cortical oscillations that have been observed during tasks assessing measures of executive function ( Bollimunta, Mo, Schroeder, & Ding, 2011 ; Cavanagh & Frank, 2014 ; Cavanagh & Shackman, 2015 ; Enriquez-Geppert, Huster, Figge, & Herrmann, 2014 ), were also present during internally guided states of focus such as meditation, a result consistent with previous findings in the literature ( Aftanas & Golocheikine, 2001 ; Kerr et al., 2013 ). These findings may also suggest a functional relationship between the sources contributing to cortical mid-frontal theta activity and the broader FPCN, a network involved in maintaining top-down representations of goal states, learning, directed attention, and the regulation of spontaneous thought ( Cavanagh & Frank, 2014 ; deBettencourt, Cohen, Lee, Norman, & Turk-Browne, 2015 ). The role of cortical theta in meditation practice and the cultivation of top-down control via the enhancement of monitoring and conflict detection falls in line with the established literature regarding its specific role in learning ( Haegens et al., 2010 ; Swick & Turken, 2002 ). Cavanagh & Frank (2014) have suggested that cortical theta (4–6 Hz) oscillations may serve as a candidate mechanism by which neurons communicate top-down control over long range and broad networks. Mid-frontal theta has been proposed to function as a temporal template for organizing mid-frontal neuronal processes ( Cavanagh & Frank, 2014 ), with theta-band phase dynamics thought to entrain disparate neural systems when cognitive control is needed (e.g., through entrainment of cortical and subcortical areas via the cingulate cortex; Bollimunta, Chen, Schroeder, & Ding, 2009 ; Morecraft & Tanji, 2009). Our previous findings provide evidence in support of the claims posited by Spreng and colleagues (2012) that the maintenance of both internal and external orientations of focus may be maintained by similar cortical theta synchronization mechanisms and suggest that meditation training may target the neural substrates underlying these oscillations.
Spontaneous fluctuations between two distinct and supposedly opposite modes during resting-state brain activity have been observed ( Fransson, 2005 ). One of these modes is characterized by the presence of slow theta oscillations, a cortical activity associated with reduced levels of vigilance. The other mode is characterized by the presence of fast oscillations of 12–30 Hz, which are usually associated with high vigilance levels ( Laufs et al., 2003 ). These spontaneous patterns of increased and decreased theta activity have been associated with periods of mind-wandering and periods of concentration, as shown in a study by Braboszcz and Delorme (2011) . During a breath awareness counting task in which subjects used the self-report method to indicate mind-wandering events, they showed an increase in occipitoparietal theta and frontocentral delta (1–3 Hz) during mind-wandering, and suggest that these findings may reflect the increased BOLD activity observed in fMRI studies investigating the DMN ( Braboszcz & Delorme, 2011 ). A functional relationship between cortical phase-locking and fluctuations in endogenous attentional states has been suggested by investigations examining the impact of training in focused attention meditation on the degree of cortical phase-locking to stimuli presentations in sustained attention tasks ( Lutz et al., 2009 ; Slagter et al., 2008 ). Lutz and colleagues (2009) found that three months of focused meditation training resulted in a smaller attentional blink and reduced brain-resource allocation to the first target (T1), demonstrated by a significantly smaller T1-elicited P3b (i.e. a neural index of resource allocation after training). Subjects with the largest decrease in cognitive resource allocation to T1 showed the largest reduction in the measured attentional-blink size, suggesting that an ability to accurately identify T2 depends upon the efficient deployment of cognitive resources to T1.
The authors hypothesized that the mental training induced increases in phase-locking were related to the capacity to sustain task-related attentional focus and a reduced tendency to engage in task-unrelated thoughts. It may be that long term meditation practice engages both top-down mechanisms underlying sustained attention as well as bottom-up processing of distracting sensory or thought related information ( Lutz et al., 2008 ). In a separate study, long-term Tibetan Nyingmapa and Kagyupa Buddhist practitioners were able to self-induce sustained high-amplitude gamma-band (25–42 Hz) oscillations and phase-synchrony, most notably over the lateral frontoparietal electrodes ( Lutz, Greischar, Rawlings, Ricard, & Davidson, 2004 ). Interestingly, in a study by Baird and colleagues (2014), they explored the sensory decoupling that occurs during mind-wandering, and whether it was mediated by the phase of ongoing cortical oscillations across one or more frequencies ( Baird, Smallwood, Lutz, & Schooler, 2014 ). This was done by analyzing the impact of task-unrelated thought on phase of cortical activity to sensory stimuli during a vigilance task, wherein a time-frequency analysis of the oscillatory neural response revealed a decrease in theta-band cortical phase-locking, which peaked over parietal scalp regions.
Recent findings published by Braboszcz and colleagues (2017) compared practitioners of three different meditation traditions (Vipassana, Himalayan Yoga, and Isha Shoonya) with a control group during a meditative and instructed mind-wandering condition, and found that all meditators showed higher parieto-occipital 60–110 Hz gamma amplitude than control subjects as a trait effect observed during meditation and when considering meditation and instructed mind-wandering periods together. Moreover, this gamma power was positively correlated with participants’ meditation experience. Additionally, they controlled for the potential contamination of muscle artifact and studied artifact activity in different experimental conditions using independent component analysis ( Braboszcz et al., 2017 ; Delorme and Makeig, 2004; >Delorme et al., 2007). Cahn and colleagues (2010) found that the cross-experimental session occipital gamma power was significantly larger in meditators with more than 10 years of daily practice, and that the meditation-related gamma power increase was similarly the strongest in such advanced practitioners ( Cahn, Delorme, & Polich, 2010 ). These findings suggest that long-term Vipassana meditation contributes to increased parieto-occipital gamma power related to long-term meditational expertise, and lend support to the link between meditation practice and increased EEG coherence (thought to facilitate the central executive functions of cognitive control and working memory; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005 ). This in turn may result in the self-regulation of lower level elements of neurogenesis ( Vago & Silbersweig, 2012 ), increased cognitive flexibility ( Slagter et al., 2011 , 2008 ), and efficient distribution of limited brain resources ( Baars, 2005 ; Lutz et al., 2008 , 2009 ).
Another intriguing finding emerging from the field of contemplative neuroscience involves the mediating role of contemplative and meditative practices on the neural mechanisms underlying sensory perception. In a study using magnetoencephalography (MEG) recording of the somatosensory cortex finger representation, Kerr and colleagues (2011) found that experienced meditators showed an enhanced alpha power modulation in response to a cue, potentially reflecting an enhanced filtering of inputs to primary sensory cortex. They also found that experienced meditators demonstrated modified alpha rhythm properties and an increase in non-localized tonic alpha power when compared to controls. An electroencephalography (EEG) study by Braboszcz and Delorme (2011) showed enhanced cortical processing of sensory stimuli during a sustained breath-focus task when compared to periods of time in which subjects reported mind-wandering. Known as the perceptual decoupling hypothesis, behavioral and neurocognitive evidence indicates that when mental events arise that are unrelated to perception they are frequently associated with a decoupling of attention from perception (Schooler et al., 2011), and that changes in spontaneous thought and mind-wandering can be either coupled or decoupled from exogenous and external perceptual events in the surrounding environment (reflecting the extent to which an individual can constrain mind-wandering; Smallwood & Schooler, 2015 ). Interestingly, Whitmarsh and colleagues (2014) investigated participant’s metacognitive ability to report on their attentional focus, and found that a contralateral somatosensory alpha power decrease was correlated with higher reported attentional focus to either their left or right hand respectively ( Whitmarsh, Barendregt, Schoffelen, & Jensen, 2014 ). Enhanced body awareness was also found to be associated with greater subjective emotional experience and awareness of heart beats during exposure to emotionally provocative stimuli in Vipassana meditators, when compared to expert dancers, and controls ( Sze et al., 2010 ). These findings can most likely be attributed to the emphasis on somatic attention training in mindfulness meditation techniques in which individuals train to develop both interoceptive awareness and metacognition; a process in which one cultivates an awareness and understanding of one’s own thought processes, and to an overall somatosensory awareness of physical sensations, feelings, and thought content ( Bishop et al., 2004 ; Farb et al., 2007 ; Segal, Teasdale, & Williams, 2004 ). Interestingly, Baird and colleagues (2014) found that a 2-week meditation program led to significantly enhanced metacognitive judgments of cognition on a trial-by-trial basis in the domain of memory, but not for perceptual decisions, suggesting that while only 2 weeks of meditation training can enhance certain elements of introspective acuity, such improvements may not apply equally to all cognitive domains, or at least may require more than 2 weeks of meditation practice.
Perspectives
Throughout this manuscript we provide a comprehensive neurocognitive framework outlining the central role of spontaneous thought processes in the meditative cycle, however many outstanding questions remain. While research findings suggest that accumulative meditation practice reduces the frequency of spontaneous thought episodes ( Brandmeyer & Delorme, 2018 ; Wenk-Sormaz, 2005 ; Zanesco et al., 2016 ), it has yet to be established whether reductions in mind wandering reflect the meta-cognitive accuracy cultivated during practice, or an increased allocation of attentional resources (improved sustained attention), or both. It may also be the case that meditation practice facilitates the unification of various attentional mechanisms so as to further mitigate mind-wandering. It is our perspective that it is most likely the case that meditative experience enhances metacognitive skills from the onset increasing an individual’s propensity to detect spontaneous thought. This would occur through the initial and repetitious «flexing» of the cognitive activity associated with the meditative cycle, with an emphasis placed on detecting when attention has drifted away from the meditative focus and bringing it back to the object (see Fig. 2 ). With accumulative practice this leads to earlier detection and sensitivity to the occurrence of mind wandering, eventually facilitating lower level neurogenesis and neuroplasticity that translate to faculties such as a the capacity for longer periods of sustained attention.
One way of deciphering the differences between this meta-cognition vs. improved attention regulation question would be to use both self- (assessing the meta-awareness of mind wandering) and probe-caught (reflecting a more direct measure of on- or off-task measure of sustained attention) experiential sampling methods. Several studies have effectively implemented this methodology in a normal population to illuminate the relationship between mind-wandering and meta-awareness. This approach was initially used to examine mind-wandering while reading and revealed that whereas participants caught themselves mind-wandering roughly 4 times in a 45 min period, they were regularly caught mind-wandering in about 15% of experience sampling probes ( Franklin, Smallwood, & Schooler, 2011 ; Unsworth & McMillan, 2013 ). This type of paradigm could be applied to meditators, and a regression analysis could be used to investigate whether the impact of years of experience influence changes in meta awareness and measures of sustained attention.
It is also important to address how using experiential sampling methods may influence the depth and quality of meditation practice in experimental settings. Interviews of meditators in experimental contexts indicate that the majority of participants are unable to experience particularly ‘deep’ meditative states, however advanced practitioners report reduced disruption from the experience sampling probes compared to novices ( Brandmeyer & Delorme, 2018 ). However, all meditators in the study experienced a progressive ‘increase in the depth’ of their meditation over time suggesting they were progressively better at engaging their meditation practice and performing the task simultaneously (there was a 10 min period of training prior to the beginning of the task). It has also been argued that all of the aforementioned methods of measurement predominantly address the contents of thought at a specific point in time, but elucidate very little regarding the neural dynamics leading up to the measurement of a given mental state (i.e., mind wandering or meditation; Smallwood & Schooler, 2015 ). Thus, nuanced methodologies such as machine learning and online classification are necessary to understand how mental states are related and evolve over time, without having to rely on subjective first person reporting ( Girn et al., 2017 ).
Another open research question is to determine to what degree different types of meditation practice influence the awareness, duration, and frequency of mind-wandering episodes. Although meditation research is a relatively established scientific domain within both academic and clinical contexts, as mentioned, much debate regarding incompatible definitions and conceptions of mindfulness and meditation remain within both academic and traditional Buddhist contexts ( Brandmeyer, Delorme, & Wahbeh, 2019 ; Dunne, 2015 ; Sharf, 2015 ; Van Dam et al., 2018 ). Similarly, the manner in which mind-wandering is defined directly influences the corresponding neuroimaging data, thus future research should aim to differentiate the distinctive forms of spontaneous cognition at both a behavioral, phenomenological, and neural level ( Fox & Christoff, 2018 ). Lastly, refined phenomenological research has yet to explore whether meditation experience exerts a preferential influence on the real time dynamics of thought content, such as the orientation (past, present, future) and the valence (pleasant, neutral, or unpleasant) of spontaneous thought processes during meditation, as well as during daily life. These findings would be particularly beneficial in therapeutic intervention trajectories, as well as in informing the broader translational and clinical applications.
One translational application of this intersection of meditation and mind wandering research includes the development of closed-loop neurofeedback paradigms. Furthermore, protocols that implement novel features including the covert detection of mind-wandering during meditation and alternative modalities of sensory feedback such as haptic feedback (as opposed to auditory or visual feedback) should be explored, as they may enable practitioners to remain in a relatively natural meditative state. Methodological approaches implementing closed-loop neurofeedback and machine learning for training meditation and detecting mind wandering have already emerged ( Bixler & D’Mello, 2016 ; Faber, Bixler, & D’Mello, 2018 ; Hutt et al., 2019 ; Krasich et al., 2018 ). While no definitive scientific consensus has been reached in terms of identifying generalizable and reliable neural markers that can serve to index meditative states and depth for neurofeedback training purposes, our previous research ( Brandmeyer, 2017 ) found behavioral improvements on a working memory task after only 8 sessions of FM theta closed-loop neurofeedback wherein subjects applied focused attention meditation instructions while upregulating frontal midline theta. Additional research by Lutterveld and Brewer (2017) found a high moment-to-moment correspondence between neurofeedback source-localized gamma activity from the PCC and subjective experience of effortless awareness. Another recent study found that when patients engaged in the Muse meditation neurofeedback (a wearable EEG head-band device and application that implements proprietary algorithms) for 20 minutes daily during the period ranging from breast cancer diagnosis until 3 months after surgical treatment, they observed reduced fatigue and reduced stress as well as an improved quality of life ( Millstine et al., 2019 ; Bhayee et al., 2016 ). Other methodological approaches such as the use of pupillometry and eye tracking during sustained visual attention tasks, the use of respiration and heart-rate variability (HRV) for assessing the down regulation of the sympathetic nervous system. New and novel methods that examine the causal role of brain regions, such as the lateral PFC, MCC, dorsal ACC, and PCC should all be considered potential candidates of feedback based on the literature presented throughout this manuscript, as these regions are heavily implicated in the meditative cycle and hold promise as candidates for future closed-loop neuro- and biofeedback protocols and applications.
Conclusions
Individual differences in executive functions and cognitive control have been shown to predict well-being across a broad array of personal, academic, and professional domains ( Hirsh & Inzlicht, 2010 ), with impaired cognitive control (i.e., impaired attention and emotion regulation) considered to be the hallmark predictor of clinical disorders such as ADHD, obsessive compulsive disorder, schizophrenia, depression, and anxiety ( Cho, Konecky, & Carter, 2006 ; Friedman et al., 2007 ; LeMoult & Gotlib, 2019 ; Mazaheri et al., 2014 ; Snyder, Kaiser, Warren, & Heller, 2015 ; Yordanova, Kolev, & Rothenberger, 2013 ). The findings presented throughout this manuscript provide a comprehensive theoretical framework showcasing the processes underlying self-generated thought and the key role that metacognitive awareness plays in enhancing cognitive regulation and which may serve to potentially buffer individuals against a wide array of cognitive deficiencies that arise from the inability to regulate self-generated thought content (i.e. rumination, habitual thinking). Many of the benefits resulting from contemplative practice are likely the result of an increased engagement with the neural circuity underlying the regulation of attention, emotions, cognition, somatosensory processing, and metacognition. Here, we highlighted how spontaneous thought processes are likely mediated by brain networks functionally and structurally linked to the practice of meditation, having focused on broadly distributed networks including the DMN, fPCN, DAN, and the salience network. We explored the key mechanisms by which meditation appears to modulate attention and sensory perception, meta-awareness, perceptual coupling, and decoupling, all of which are directly implicated in the regulation, maintenance, content, valence, and overall generation of spontaneous thought processes, broader cognitive functioning, and well-being.
Akwnowledgments
This work was supported by grants from the Agence Nationale pour la Recherche (ANR-12-JSH2-0009), the BIAL foundation (BIAL-08-162), and a T32 award (T32 AT00399) granted at the University of California San Francisco, Osher Center for Integrative Medicine from the National Center for Complementary and Integrative Health of the National Institutes of Health. The authors wish to thank Cédric Cannard and Phillippe Goldin for their contributions towards the manuscript.
- Addis DR, Knapp K, Roberts RP, & Schacter DL (2012). Routes to the past: Neural substrates of direct and generative autobiographical memory retrieval . NeuroImage , 59 ( 3 ), 2908–2922. 10.1016/j.neuroimage.2011.09.066 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aftanas LI, & Golocheikine SA (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation . Neuroscience Letters , 310 ( 1 ), 57–60. 10.1016/S0304-3940(01)02094-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ainsworth B, Eddershaw R, Meron D, Baldwin DS, & Garner M (2013). The effect of focused attention and open monitoring meditation on attention network function in healthy volunteers . Psychiatry Research , 210 ( 3 ), 1226–1231. 10.1016/j.psychres.2013.09.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amodio DM, & Frith CD (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews . Neuroscience , 7 ( 4 ), 268–277. 10.1038/nrn1884 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andrews-Hanna JR, Irving ZC, Fox KCR, Spreng RN, & Christoff K (2017). The Neuroscience of Spontaneous Thought: An Evolving, Interdisciplinary Field . ArXiv:1704.02533 [q-Bio] . Retrieved from http://arxiv.org/abs/1704.02533 [ Google Scholar ]
- Andrews-Hanna JR, Kaiser RH, Turner AEJ, Reineberg A, Godinez D, Dimidjian S, & Banich M (2013). A penny for your thoughts: Dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing . Frontiers in Psychology , 4 10.3389/fpsyg.2013.00900 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the Default Network’s Role in Spontaneous Cognition . Journal of Neurophysiology , 104 ( 1 ), 322–335. 10.1152/jn.00830.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andrews-Hanna JR, Saxe R, & Yarkoni T (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses . NeuroImage , 91 , 324–335. 10.1016/j.neuroimage.2014.01.032 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance . Annals of the New York Academy of Sciences , 1316 ( 1 ), 29–52. 10.1111/nyas.12360 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, & Krystal JH (2012). The role of default network deactivation in cognition and disease . Trends in Cognitive Sciences , 16 ( 12 ), 584–592. 10.1016/j.tics.2012.10.008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baars BJ (2005). Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience . Progress in Brain Research , 150 , 45–53. 10.1016/S0079-6123(05)50004-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baer RA (2015). Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications . Elsevier. [ Google Scholar ]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, & Toney L (2006). Using Self-Report Assessment Methods to Explore Facets of Mindfulness . Assessment , 13 ( 1 ), 27–45. 10.1177/1073191105283504 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baird B, Mrazek MD, Phillips DT, & Schooler JW (2014). Domain-specific enhancement of metacognitive ability following meditation training . Journal of Experimental Psychology: General , 143 ( 5 ), 1972–1979. 10.1037/a0036882 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baird B, Smallwood J, Gorgolewski KJ, & Margulies DS (2013). Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience , 33 ( 42 ), 16657–16665. 10.1523/JNEUROSCI.0786-13.2013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baird B, Smallwood J, Lutz A, & Schooler JW (2014). The Decoupled Mind: Mind-wandering Disrupts Cortical Phase-locking to Perceptual Events . Journal of Cognitive Neuroscience , 26 , 2596–2607. 10.1162/jocn_a_00656 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baird B, Smallwood J, Mrazek MD, Kam JWY, Franklin MS, & Schooler JW (2012). Inspired by Distraction: Mind Wandering Facilitates Creative Incubation . Psychological Science , 23 ( 10 ), 1117–1122. 10.1177/0956797612446024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baird B, Smallwood J, & Schooler JW (2011). Back to the future: Autobiographical planning and the functionality of mind-wandering . Consciousness and Cognition , 20 ( 4 ), 1604–1611. 10.1016/j.concog.2011.08.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks JB, Welhaf MS, & Srour A (2015). The protective effects of brief mindfulness meditation training . Consciousness and Cognition , 33 , 277–285. 10.1016/j.concog.2015.01.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barron E, Riby LM, Greer J, & Smallwood J (2011). Absorbed in Thought: The Effect of Mind Wandering on the Processing of Relevant and Irrelevant Events . Psychological Science , 22 ( 5 ), 596–601. 10.1177/0956797611404083 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network . Social Cognitive and Affective Neuroscience , 6 ( 5 ), 548–555. 10.1093/scan/nsq080 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhayee S, Tomaszewski P, Lee DH, Moffat G, Pino L, Moreno S, & Farb NAS (2016). Attentional and affective consequences of technology supported mindfulness training: A randomised, active control, efficacy trial . BMC Psychology , 4 ( 1 ), 60 10.1186/s40359-016-0168-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, … Devins G (2004). Mindfulness: A Proposed Operational Definition . Clinical Psychology: Science and Practice , 11 ( 3 ), 230–241. 10.1093/clipsy.bph077 [ CrossRef ] [ Google Scholar ]
- Bixler R, & D’Mello S (2016). Automatic gaze-based user-independent detection of mind wandering during computerized reading . User Modeling and User-Adapted Interaction , 26 ( 1 ), 33–68. 10.1007/s11257-015-9167-1 [ CrossRef ] [ Google Scholar ]
- Bockelman P, Reinerman-Jones L, & Gallagher S (2013). Methodological lessons in neurophenomenology: Review of a baseline study and recommendations for research approaches . Frontiers in Human Neuroscience , 7. 10.3389/fnhum.2013.00608 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bollimunta A, Chen Y, Schroeder CE, & Ding M (2009). Characterizing Oscillatory Cortical Networks with Granger Causality . In Josic K, Rubin J, Matias M, & Romo R (Eds.), Coherent Behavior in Neuronal Networks (pp. 169–189). 10.1007/978-1-4419-0389-1_9 [ CrossRef ] [ Google Scholar ]
- Bollimunta A, Mo J, Schroeder CE, & Ding M (2011). Neuronal Mechanisms and Attentional Modulation of Corticothalamic Alpha Oscillations . Journal of Neuroscience , 31 ( 13 ), 4935–4943. 10.1523/JNEUROSCI.5580-10.2011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Braboszcz C, Cahn BR, Levy J, Fernandez M, & Delorme A (2017). Increased Gamma Brainwave Amplitude Compared to Control in Three Different Meditation Traditions . PLOS ONE , 12 ( 1 ), e0170647 10.1371/journal.pone.0170647 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Braboszcz C, & Delorme A (2011). Lost in thoughts: Neural markers of low alertness during mind wandering . NeuroImage , 54 ( 4 ), 3040–3047. 10.1016/j.neuroimage.2010.10.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brandmeyer T (2017). Investigating the role of oscillations in endogenous and exogenous attentional states: Novel methods in neurophenomenology (Phdthesis, Université Paul Sabatier - Toulouse III). Retrieved from https://tel.archives-ouvertes.fr/tel-01772802/document [ Google Scholar ]
- Brandmeyer T, & Delorme A (2018). Reduced mind wandering in experienced meditators and associated EEG correlates . Experimental Brain Research , 236 ( 9 ), 2519–2528. 10.1007/s00221-016-4811-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brandmeyer T, Delorme A, & Wahbeh H (2019). The neuroscience of meditation: Classification, phenomenology, correlates, and mechanisms . Progress in Brain Research , 244 , 1–29. 10.1016/bs.pbr.2018.10.020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, & Davidson RJ (2007). Neural correlates of attentional expertise in long-term meditation practitioners . Proceedings of the National Academy of Sciences , 104 ( 27 ), 11483–11488. 10.1073/pnas.0606552104 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, & Kober H (2011). Meditation experience is associated with differences in default mode network activity and connectivity . Proceedings of the National Academy of Sciences of the United States of America , 108 ( 50 ), 20254–20259. 10.1073/pnas.1112029108 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown KW, & Ryan RM (2003). The benefits of being present: Mindfulness and its role in psychological well-being . Journal of Personality and Social Psychology , 84 ( 4 ), 822–848. 10.1037/0022-3514.84.4.822 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown Kirk Warren, & Ryan RM (2004). Perils and Promise in Defining and Measuring Mindfulness: Observations From Experience . Clinical Psychology: Science and Practice , 11 ( 3 ), 242–248. 10.1093/clipsy.bph078 [ CrossRef ] [ Google Scholar ]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease . Annals of the New York Academy of Sciences , 1124 , 1–38. 10.1196/annals.1440.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burgess PW, Dumontheil I, & Gilbert SJ (2007). The gateway hypothesis of rostral prefrontal cortex (area 10) function . Trends in Cognitive Sciences , 11 ( 7 ), 290–298. 10.1016/j.tics.2007.05.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahn BR, Delorme A, & Polich J (2010). Occipital gamma activation during Vipassana meditation . Cognitive Processing , 11 ( 1 ), 39–56. 10.1007/s10339-009-0352-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahn BR, & Polich J (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies . Psychological Bulletin , 132 ( 2 ), 180–211. 10.1037/0033-2909.132.2.180 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carter KS, & Carter III R (2016). Breath-based meditation: A mechanism to restore the physiological and cognitive reserves for optimal human performance . World Journal of Clinical Cases , 4 ( 4 ), 99–102. 10.12998/wjcc.v4.i4.99 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control . Trends in Cognitive Sciences , 18 ( 8 ), 414–421. 10.1016/j.tics.2014.04.012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence . Journal of Physiology-Paris , 109 ( 1 ), 3–15. 10.1016/j.jphysparis.2014.04.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chan D, & Woollacott M (2007). Effects of Level of Meditation Experience on Attentional Focus: Is the Efficiency of Executive or Orientation Networks Improved? The Journal of Alternative and Complementary Medicine , 13 ( 6 ), 651–658. 10.1089/acm.2007.7022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chiesa A, & Malinowski P (2011). Mindfulness-based approaches: Are they all the same? Journal of Clinical Psychology , 67 ( 4 ), 404–424. 10.1002/jclp.20776 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cho RY, Konecky RO, & Carter CS (2006). Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia . Proceedings of the National Academy of Sciences , 103 ( 52 ), 19878–19883. 10.1073/pnas.0609440103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering . Proceedings of the National Academy of Sciences , 106 ( 21 ), 8719–8724. 10.1073/pnas.0900234106 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Christoff K, Irving ZC, Fox KCR, Spreng RN, & Andrews-Hanna JR (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews . Neuroscience , 17 ( 11 ), 718–731. 10.1038/nrn.2016.113 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Christoff K, Ream JM, Geddes LPT, & Gabrieli JDE (2003). Evaluating Self-Generated Information: Anterior Prefrontal Contributions to Human Cognition . Behavioral Neuroscience , 117 ( 6 ), 1161–1168. 10.1037/0735-7044.117.6.1161 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Colzato LS, Szapora A, & Hommel B (2012a). Meditate to Create: The Impact of Focused-Attention and Open-Monitoring Training on Convergent and Divergent Thinking . Frontiers in Psychology , 3 10.3389/fpsyg.2012.00116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Colzato LS, Szapora A, & Hommel B (2012b). Meditate to Create: The Impact of Focused-Attention and Open-Monitoring Training on Convergent and Divergent Thinking . Frontiers in Psychology , 3 10.3389/fpsyg.2012.00116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body . Nature Reviews Neuroscience , 3 ( 8 ), 655–666. 10.1038/nrn894 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Creswell JD, & Lindsay EK (2014). How Does Mindfulness Training Affect Health? A Mindfulness Stress Buffering Account . Current Directions in Psychological Science , 23 ( 6 ), 401–407. 10.1177/0963721414547415 [ CrossRef ] [ Google Scholar ]
- Curtis CE, & D’Esposito M (2003). Persistent activity in the prefrontal cortex during working memory . Trends in Cognitive Sciences , 7 ( 9 ), 415–423. 10.1016/S1364-6613(03)00197-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dahl CJ, Lutz A, & Davidson RJ (2015). Reconstructing and deconstructing the self: Cognitive mechanisms in meditation practice . Trends in Cognitive Sciences , 19 ( 9 ), 515–523. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- D’Argembeau A, Renaud O, & Linden MV der. (2011). Frequency, characteristics and functions of future-oriented thoughts in daily life . Applied Cognitive Psychology , 25 ( 1 ), 96–103. 10.1002/acp.1647 [ CrossRef ] [ Google Scholar ]
- Davidson RJ (2010). Empirical explorations of mindfulness: Conceptual and methodological conundrums . Emotion , 10 ( 1 ), 8–11. 10.1037/a0018480 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- deBettencourt MT, Cohen JD, Lee RF, Norman KA, & Turk-Browne NB (2015). Closed-loop training of attention with real-time brain imaging . Nature Neuroscience , 18 ( 3 ), 470–475. 10.1038/nn.3940 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delorme A, & Brandmeyer T (2019). When the meditating mind wanders . Current Opinion in Psychology , 28 , 133–137. 10.1016/j.copsyc.2018.12.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deng Y-Q, Li S, & Tang Y-Y (2014). The Relationship Between Wandering Mind, Depression and Mindfulness . Mindfulness , 5 ( 2 ), 124–128. 10.1007/s12671-012-0157-7 [ CrossRef ] [ Google Scholar ]
- Desbordes G, Gard T, Hoge EA, Hölzel B, Kerr C, Lazar SW, … Vago DR (2015). Moving Beyond Mindfulness: Defining Equanimity as an Outcome Measure in Meditation and Contemplative Research . Mindfulness , 6 ( 2 ), 356–372. 10.1007/s12671-013-0269-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Desimone R, & Duncan J (1995). Neural Mechanisms of Selective Visual Attention . Annual Review of Neuroscience , 18 ( 1 ), 193–222. 10.1146/annurev.ne.18.030195.001205 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D’Esposito M (2007). From cognitive to neural models of working memory . Philosophical Transactions of the Royal Society B: Biological Sciences , 362 ( 1481 ), 761–772. 10.1098/rstb.2007.2086 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dixon ML, Fox KCR, & Christoff K (2014). A framework for understanding the relationship between externally and internally directed cognition . Neuropsychologia , 62 , 321–330. 10.1016/j.neuropsychologia.2014.05.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dixon ML, Vega ADL, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, & Christoff K (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks . Proceedings of the National Academy of Sciences , 115 ( 7 ), E1598–E1607. 10.1073/pnas.1715766115 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, & Sorg C (2015). Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks . Frontiers in Human Neuroscience , 9 10.3389/fnhum.2015.00461 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dorjee D (2016). Defining Contemplative Science: The Metacognitive Self-Regulatory Capacity of the Mind, Context of Meditation Practice and Modes of Existential Awareness . Frontiers in Psychology , 7 10.3389/fpsyg.2016.01788 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dresler M, Wehrle R, Spoormaker VI, Koch SP, Holsboer F, Steiger A, … Czisch M (2012). Neural correlates of dream lucidity obtained from contrasting lucid versus non-lucid REM sleep: A combined EEG/fMRI case study . Sleep , 35 ( 7 ), 1017–1020. 10.5665/sleep.1974 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dunne JD (2015). Buddhist Styles of Mindfulness: A Heuristic Approach . In Ostafin BD, Robinson MD, & Meier BP (Eds.), Handbook of Mindfulness and Self-Regulation (pp. 251–270). 10.1007/978-1-4939-2263-5_18 [ CrossRef ] [ Google Scholar ]
- Ehlers A, Hackmann A, & Michael T (2004). Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy . Memory , 12 ( 4 ), 403–415. 10.1080/09658210444000025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ellamil M, Fox KCR, Dixon ML, Pritchard S, Todd RM, Thompson E, & Christoff K (2016). Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners . NeuroImage , 136 , 186–196. 10.1016/j.neuroimage.2016.04.034 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enriquez-Geppert S, Huster RJ, Figge C, & Herrmann CS (2014). Self-regulation of frontal-midline theta facilitates memory updating and mental set shifting . Frontiers in Behavioral Neuroscience , 8 10.3389/fnbeh.2014.00420 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, & Mendes WB (2013). Wandering Minds and Aging Cells . Clinical Psychological Science , 1 ( 1 ), 75–83. 10.1177/2167702612460234 [ CrossRef ] [ Google Scholar ]
- Evans DR, & Segerstrom SC (2011). Why do Mindful People Worry Less? Cognitive Therapy and Research , 35 ( 6 ), 505–510. 10.1007/s10608-010-9340-0 [ CrossRef ] [ Google Scholar ]
- Faber M, Bixler R, & D’Mello SK (2018). An automated behavioral measure of mind wandering during computerized reading . Behavior Research Methods , 50 ( 1 ), 134–150. 10.3758/s13428-017-0857-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, & Anderson AK (2007). Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference . Social Cognitive and Affective Neuroscience , 2 ( 4 ), 313–322. 10.1093/scan/nsm030 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Filevich E, Dresler M, Brick TR, & Kühn S (2015). Metacognitive mechanisms underlying lucid dreaming. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience , 35 ( 3 ), 1082–1088. 10.1523/JNEUROSCI.3342-14.2015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fleming SM, & Dolan RJ (2012). The neural basis of metacognitive ability . Phil. Trans. R. Soc. B , 367 ( 1594 ), 1338–1349. 10.1098/rstb.2011.0417 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fleming SM, Huijgen J, & Dolan RJ (2012). Prefrontal Contributions to Metacognition in Perceptual Decision Making . Journal of Neuroscience , 32 ( 18 ), 6117–6125. 10.1523/JNEUROSCI.6489-11.2012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, & Rees G (2010). Relating Introspective Accuracy to Individual Differences in Brain Structure . Science , 329 ( 5998 ), 1541–1543. 10.1126/science.1191883 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Flook L, Goldberg SB, Pinger L, & Davidson RJ (2015). Promoting prosocial behavior and self-regulatory skills in preschool children through a mindfulness-based kindness curriculum . Developmental Psychology , 51 ( 1 ), 44–51. 10.1037/a0038256 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, Andrews-Hanna JR, Mills C, Dixon ML, Markovic J, Thompson E, & Christoff K (2018). Affective neuroscience of self-generated thought . Annals of the New York Academy of Sciences , 1426 ( 1 ), 25–51. 10.1111/nyas.13740 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, & Christoff K (2014). Metacognitive Facilitation of Spontaneous Thought Processes: When Metacognition Helps the Wandering Mind Find Its Way . In Fleming SM & Frith CD (Eds.), The Cognitive Neuroscience of Metacognition (pp. 293–319). 10.1007/978-3-642-45190-4_13 [ CrossRef ] [ Google Scholar ]
- Fox KCR, & Christoff K (2018). The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming . Oxford University Press. [ Google Scholar ]
- Fox KCR, Dixon ML, Nijeboer S, Girn M, Floman JL, Lifshitz M, … Christoff K (2016). Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations . Neuroscience & Biobehavioral Reviews , 65 , 208–228. 10.1016/j.neubiorev.2016.03.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, … Christoff K (2014). Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners . Neuroscience & Biobehavioral Reviews , 43 , 48–73. 10.1016/j.neubiorev.2014.03.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, Nijeboer S, Solomonova E, Domhoff GW, & Christoff K (2013). Dreaming as mind wandering: Evidence from functional neuroimaging and first-person content reports . Frontiers in Human Neuroscience , 7 10.3389/fnhum.2013.00412 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, & Christoff K (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes . NeuroImage , 111 , 611–621. 10.1016/j.neuroimage.2015.02.039 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox KCR, Zakarauskas P, Dixon M, Ellamil M, Thompson E, & Christoff K (2012). Meditation Experience Predicts Introspective Accuracy . PLOS ONE , 7 ( 9 ), e45370 10.1371/journal.pone.0045370 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Franklin MS, Mrazek MD, Anderson CL, Smallwood J, Kingstone A, & Schooler J (2013). The silver lining of a mind in the clouds: Interesting musings are associated with positive mood while mind-wandering . Frontiers in Psychology , 4 10.3389/fpsyg.2013.00583 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Franklin MS, Smallwood J, & Schooler JW (2011). Catching the mind in flight: Using behavioral indices to detect mindless reading in real time . Psychonomic Bulletin & Review , 18 ( 5 ), 992–997. 10.3758/s13423-011-0109-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fransson P (2005). Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis . Human Brain Mapping , 26 ( 1 ), 15–29. 10.1002/hbm.20113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frewen PA, Evans EM, Maraj N, Dozois DJA, & Partridge K (2008). Letting Go: Mindfulness and Negative Automatic Thinking . Cognitive Therapy and Research , 32 ( 6 ), 758–774. 10.1007/s10608-007-9142-1 [ CrossRef ] [ Google Scholar ]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, & Hewitt JK (2007). Greater Attention Problems During Childhood Predict Poorer Executive Functioning in Late Adolescence . Psychological Science , 18 ( 10 ), 893–900. 10.1111/j.1467-9280.2007.01997.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, … Sobin P (2012a). Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond . Evidence-Based Complementary and Alternative Medicine: ECAM , 2012 , 680407 10.1155/2012/680407 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, … Sobin P (2012b). Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond [Research article] . 10.1155/2012/680407 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garrison KA, Scheinost D, Constable RT, & Brewer JA (2014). BOLD signal and functional connectivity associated with loving kindness meditation . Brain and Behavior , 4 ( 3 ), 337–347. 10.1002/brb3.219 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, & Brewer JA (2015). Meditation leads to reduced default mode network activity beyond an active task . Cognitive, Affective, & Behavioral Neuroscience , 15 ( 3 ), 712–720. 10.3758/s13415-015-0358-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Girn M, Mills C, Laycock E, Ellamil M, Ward L, & Christoff K (2017). Neural Dynamics of Spontaneous Thought: An Electroencephalographic Study . In Schmorrow DD & Fidopiastis CM (Eds.), Augmented Cognition. Neurocognition and Machine Learning (Vol. 10284 , pp. 28–44). 10.1007/978-3-319-58628-1_3 [ CrossRef ] [ Google Scholar ]
- Gold SR, & Gold RG (1982). Actual daydream content and the Imaginal Processes Inventory . Journal of Mental Imagery , 6 ( 1 ), 169–173. [ Google Scholar ]
- Goldin PR, & Gross JJ (2010). Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder . Emotion (Washington, D.C.) , 10 ( 1 ), 83–91. 10.1037/a0018441 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis . JAMA Internal Medicine , 174 ( 3 ), 357–368. 10.1001/jamainternmed.2013.13018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gusnard DA, Raichle ME, & Raichle ME (2001). Searching for a baseline: Functional imaging and the resting human brain . Nature Reviews. Neuroscience , 2 ( 10 ), 685–694. 10.1038/35094500 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haegens S, Osipova D, Oostenveld R, & Jensen O (2010). Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network . Human Brain Mapping , 31 ( 1 ), 26–35. 10.1002/hbm.20842 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, & Shibasaki H (2002). The Role of Rostral Brodmann Area 6 in Mental-operation Tasks: An Integrative Neuroimaging Approach . Cerebral Cortex , 12 ( 11 ), 1157–1170. 10.1093/cercor/12.11.1157 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hanley AW, & Garland EL (2014). Dispositional Mindfulness Co-varies with Self-Reported Positive Reappraisal . Personality and Individual Differences , 66 , 146–152. 10.1016/j.paid.2014.03.014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hart W (2011). The Art of Living: Vipassana Meditation as Taught by S.N. Goenka. Pariyatti .
- Hasenkamp W, & Barsalou LW (2012). Effects of meditation experience on functional connectivity of distributed brain networks . Frontiers in Human Neuroscience , 6 , 38 10.3389/fnhum.2012.00038 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, & Barsalou LW (2012). Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states . NeuroImage , 59 ( 1 ), 750–760. 10.1016/j.neuroimage.2011.07.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hirsh JB, & Inzlicht M (2010). Error-related negativity predicts academic performance . Psychophysiology, 47 ( 1 ), 192–196. 10.1111/j.1469-8986.2009.00877.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hodgins HS, & Adair KC (2010). Attentional processes and meditation . Consciousness and Cognition , 19 ( 4 ), 872–878. 10.1016/j.concog.2010.04.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hofmann SG, Grossman P, & Hinton DE (2011). Loving-kindness and compassion meditation: Potential for psychological interventions . Clinical Psychology Review , 31 ( 7 ), 1126–1132. 10.1016/j.cpr.2011.07.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation . Trends in Cognitive Sciences , 16 ( 3 ), 174–180. 10.1016/j.tics.2012.01.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, & Ott U (2011). How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective . Perspectives on Psychological Science: A Journal of the Association for Psychological Science , 6 ( 6 ), 537–559. 10.1177/1745691611419671 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hutt S, Krasich K, Mills C, Bosch N, White S, Brockmole JR, & D’Mello SK (2019). Automated gaze-based mind wandering detection during computerized learning in classrooms . User Modeling and User-Adapted Interaction . 10.1007/s11257-019-09228-5 [ CrossRef ] [ Google Scholar ]
- Irving ZC (2016). Mind-wandering is unguided attention: Accounting for the “purposeful” wanderer . Philosophical Studies , 173 ( 2 ), 547–571. 10.1007/s11098-015-0506-1 [ CrossRef ] [ Google Scholar ]
- Jang JH, Jung WH, Kang D-H, Byun MS, Kwon SJ, Choi C-H, & Kwon JS (2011). Increased default mode network connectivity associated with meditation . Neuroscience Letters , 487 ( 3 ), 358–362. 10.1016/j.neulet.2010.10.056 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jazaieri H, Lee IA, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2016). A wandering mind is a less caring mind: Daily experience sampling during compassion meditation training . The Journal of Positive Psychology , 11 ( 1 ), 37–50. 10.1080/17439760.2015.1025418 [ CrossRef ] [ Google Scholar ]
- Jazaieri H, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2014). A randomized controlled trial of compassion cultivation training: Effects on mindfulness, affect, and emotion regulation . Motivation and Emotion , 38 ( 1 ), 23–35. 10.1007/s11031-013-9368-z [ CrossRef ] [ Google Scholar ]
- Jha AP, Krompinger J, & Baime MJ (2007). Mindfulness training modifies subsystems of attention . Cognitive, Affective, & Behavioral Neuroscience , 7 ( 2 ), 109–119. 10.3758/CABN.7.2.109 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jha AP, Morrison AB, Parker SC, & Stanley EA (2017). Practice Is Protective: Mindfulness Training Promotes Cognitive Resilience in High-Stress Cohorts . Mindfulness , 8 ( 1 ), 46–58. 10.1007/s12671-015-0465-9 [ CrossRef ] [ Google Scholar ]
- Kabat-Zinn J (2003). Mindfulness-Based Interventions in Context: Past, Present, and Future . Clinical Psychology: Science and Practice , 10 ( 2 ), 144–156. 10.1093/clipsy.bpg016 [ CrossRef ] [ Google Scholar ]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, & Kwapil TR (2007). For Whom the Mind Wanders, and When: An Experience-Sampling Study of Working Memory and Executive Control in Daily Life . Psychological Science , 18 ( 7 ), 614–621. 10.1111/j.1467-9280.2007.01948.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kerr CE, Jones SR, Wan Q, Pritchett DL, Wasserman RH, Wexler A, … Moore CI (2011). Effects of mindfulness meditation training on anticipatory alpha modulation in primary somatosensory cortex . Brain Research Bulletin , 85 ( 3 ), 96–103. 10.1016/j.brainresbull.2011.03.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, & Jones SR (2013). Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation . Frontiers in Human Neuroscience , 7 10.3389/fnhum.2013.00012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, & Tranel D (2008). Interoceptive awareness in experienced meditators . Psychophysiology , 45 ( 4 ), 671–677. 10.1111/j.1469-8986.2008.00666.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Killingsworth MA, & Gilbert DT (2010). A Wandering Mind Is an Unhappy Mind . Science , 330 ( 6006 ), 932–932. 10.1126/science.1192439 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Klein SB, Robertson TE, Delton AW, & Lax ML (2012). Familiarity and Personal Experience as Mediators of Recall When Planning for Future Contingencies . Journal of Experimental Psychology. Learning, Memory, and Cognition , 38 ( 1 ), 240–245. 10.1037/a0025200 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Klinger E (2009). Daydreaming and fantasizing: Thought flow and motivation In Handbook of imagination and mental simulation (pp. 225–239). New York, NY, US: Psychology Press. [ Google Scholar ]
- Klinger E (2013). Goal Commitments and the content of thoughts and dreams: Basic principles . Frontiers in Psychology , 4 10.3389/fpsyg.2013.00415 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Krasich K, McManus R, Hutt S, Faber M, D’Mello SK, & Brockmole JR (2018). Gaze-based signatures of mind wandering during real-world scene processing . Journal of Experimental Psychology: General , 147 ( 8 ), 1111–1124. 10.1037/xge0000411 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kucyi A, Salomons TV, & Davis KD (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks . Proceedings of the National Academy of Sciences of the United States of America , 110 ( 46 ), 18692–18697. 10.1073/pnas.1312902110 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, & Kleinschmidt A (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest . Proceedings of the National Academy of Sciences , 100 ( 19 ), 11053–11058. 10.1073/pnas.1831638100 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, & Benson H (2000). Functional brain mapping of the relaxation response and meditation . NeuroReport , 11 ( 7 ), 1581 Retrieved from https://journals.lww.com/neuroreport/Fulltext/2000/05150/Functional_brain_mapping_of_the_relaxation.42.aspx [ PubMed ] [ Google Scholar ]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, … Fischl B (2005). Meditation experience is associated with increased cortical thickness . Neuroreport , 16 ( 17 ), 1893–1897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- LeMoult J, & Gotlib IH (2019). Depression: A cognitive perspective . Clinical Psychology Review , 69 , 51–66. 10.1016/j.cpr.2018.06.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, & Nowak M (1999). A 15O-H2O PET study of meditation and the resting state of normal consciousness . Human Brain Mapping , 7 ( 2 ), 98–105. 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luders E, Toga AW, Lepore N, & Gaser C (2009). The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter . NeuroImage , 45 ( 3 ), 672–678. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3184843/ [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, & Davidson RJ (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice . Proceedings of the National Academy of Sciences , 101 ( 46 ), 16369–16373. 10.1073/pnas.0407401101 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lutz A, Slagter HA, Dunne JD, & Davidson RJ (2008). Attention regulation and monitoring in meditation . Trends in Cognitive Sciences , 12 ( 4 ), 163–169. 10.1016/j.tics.2008.01.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, & Davidson RJ (2009). Mental Training Enhances Attentional Stability: Neural and Behavioral Evidence . Journal of Neuroscience , 29 ( 42 ), 13418–13427. 10.1523/JNEUROSCI.1614-09.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lutz A, & Thompson E (2003). Neurophenomenology Integrating Subjective Experience and Brain Dynamics in the Neuroscience of Consciousness [Text]. Retrieved October 18, 2018, from https://www.ingentaconnect.com/content/imp/jcs/2003/00000010/F0020009/art00004
- MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, … Saron CD (2010). Intensive Meditation Training Improves Perceptual Discrimination and Sustained Attention . Psychological Science , 21 ( 6 ), 829–839. 10.1177/0956797610371339 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malouf ET, Youman K, Stuewig J, Witt EA, & Tangney JP (2017). A Pilot RCT of a Values-Based Mindfulness Group Intervention with Jail Inmates: Evidence for Reduction in Post-Release Risk Behavior . Mindfulness , 8 ( 3 ), 603–614. 10.1007/s12671-016-0636-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Markovic J, Anderson AK, & Todd RM (2014). Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory . Behavioural Brain Research , 259 , 229–241. 10.1016/j.bbr.2013.11.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazaheri A, Fassbender C, Coffey-Corina S, Hartanto TA, Schweitzer JB, & Mangun GR (2014). Differential Oscillatory Electroencephalogram Between Attention-Deficit/Hyperactivity Disorder Subtypes and Typically Developing Adolescents . Biological Psychiatry , 76 ( 5 ), 422–429. 10.1016/j.biopsych.2013.08.023 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McCaig RG, Dixon M, Keramatian K, Liu I, & Christoff K (2011). Improved modulation of rostrolateral prefrontal cortex using real-time fMRI training and meta-cognitive awareness . NeuroImage , 55 ( 3 ), 1298–1305. 10.1016/j.neuroimage.2010.12.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McConville J, McAleer R, & Hahne A (2017). Mindfulness Training for Health Profession Students—The Effect of Mindfulness Training on Psychological Well-Being, Learning and Clinical Performance of Health Professional Students: A Systematic Review of Randomized and Non-randomized Controlled Trials . EXPLORE , 13 ( 1 ), 26–45. 10.1016/j.explore.2016.10.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, & Pessoa L (2014). Network Organization Unfolds over Time during Periods of Anxious Anticipation . Journal of Neuroscience , 34 ( 34 ), 11261–11273. 10.1523/JNEUROSCI.1579-14.2014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McMillan R, Kaufman SB, & Singer JL (2013). Ode to positive constructive daydreaming . Frontiers in Psychology , 4 10.3389/fpsyg.2013.00626 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McVay JC, & Kane MJ (2010). Adrift in the Stream of Thought: The Effects of Mind Wandering on Executive Control and Working Memory Capacity . In Gruszka A, Matthews G, & Szymura B (Eds.), Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control (pp. 321–334). 10.1007/978-1-4419-1210-7_19 [ CrossRef ] [ Google Scholar ]
- Menezes CB, de Paula Couto MC, Buratto LG, Erthal F, Pereira MG, & Bizarro L (2013). The Improvement of Emotion and Attention Regulation after a 6-Week Training of Focused Meditation: A Randomized Controlled Trial [Research article] . 10.1155/2013/984678 [ PMC free article ] [ PubMed ] [ CrossRef ]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function . Brain Structure and Function , 214 ( 5 ), 655–667. 10.1007/s00429-010-0262-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller EK, & Cohen JD (2001). An Integrative Theory of Prefrontal Cortex Function . Annual Review of Neuroscience , 24 ( 1 ), 167–202. 10.1146/annurev.neuro.24.1.167 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Millstine DM, Bhagra A, Jenkins SM, Croghan IT, Stan DL, Boughey JC, … Pruthi S (2019). Use of a Wearable EEG Headband as a Meditation Device for Women With Newly Diagnosed Breast Cancer: A Randomized Controlled Trial . Integrative Cancer Therapies , 18 , 1534735419878770 10.1177/1534735419878770 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mooneyham BW, & Schooler JW (2013). The costs and benefits of mind-wandering: A review . Canadian Journal of Experimental Psychology = Revue Canadienne De Psychologie Experimentale , 67 ( 1 ), 11–18. 10.1037/a0031569 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moore A, & Malinowski P (2009). Meditation, mindfulness and cognitive flexibility . Consciousness and Cognition , 18 ( 1 ), 176–186. 10.1016/j.concog.2008.12.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morecraft RJ, & Tanji J (n.d.). Cingulofrontal Interactions and the Cingulate Motor Areas . 32 . [ Google Scholar ]
- Morone NE, Greco CM, & Weiner DK (2008). Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study . PAIN , 134 ( 3 ), 310–319. 10.1016/j.pain.2007.04.038 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morrison AB, Goolsarran M, Rogers SL, & Jha AP (2014). Taming a wandering attention: Short-form mindfulness training in student cohorts . Frontiers in Human Neuroscience , 7 10.3389/fnhum.2013.00897 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mrazek MD, Franklin MS, Phillips DT, Baird B, & Schooler JW (2013). Mindfulness Training Improves Working Memory Capacity and GRE Performance While Reducing Mind Wandering . Psychological Science , 24 ( 5 ), 776–781. 10.1177/0956797612459659 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mrazek MD, Smallwood J, & Schooler JW (2012). Mindfulness and mind-wandering: Finding convergence through opposing constructs . Emotion , 12 ( 3 ), 442–448. 10.1037/a0026678 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, & d’Aquili E (2001). The measurement of regional cerebral blood flow during the complex cognitive task of meditation: A preliminary SPECT study. Psychiatry Research : Neuroimaging , 106 ( 2 ), 113–122. 10.1016/S0925-4927(01)00074-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms . Journal of Abnormal Psychology , 109 ( 3 ), 504–511. [ PubMed ] [ Google Scholar ]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion . Trends in Cognitive Sciences , 9 ( 5 ), 242–249. 10.1016/j.tics.2005.03.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion . NeuroImage , 23 ( 2 ), 483–499. 10.1016/j.neuroimage.2004.06.030 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Okoro CA, Zhao G, Li C, & Balluz LS (2012). Use of complementary and alternative medicine among US adults with and without functional limitations . Disability and Rehabilitation , 34 ( 2 ), 128–135. 10.3109/09638288.2011.591887 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, … Blackburn EH (2013). Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study . The Lancet Oncology , 14 ( 11 ), 1112–1120. 10.1016/S1470-2045(13)70366-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ostafin BD, & Kassman KT (2012). Stepping out of history: Mindfulness improves insight problem solving . Consciousness and Cognition , 21 ( 2 ), 1031–1036. 10.1016/j.concog.2012.02.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ottaviani C, & Couyoumdjian A (2013). Pros and cons of a wandering mind: A prospective study . Frontiers in Psychology , 4 , 524 10.3389/fpsyg.2013.00524 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ottaviani C, Shapiro D, & Couyoumdjian A (2013). Flexibility as the key for somatic health: From mind wandering to perseverative cognition . Biological Psychology , 94 ( 1 ), 38–43. 10.1016/j.biopsycho.2013.05.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pagnoni G (2012). Dynamical Properties of BOLD Activity from the Ventral Posteromedial Cortex Associated with Meditation and Attentional Skills . Journal of Neuroscience , 32 ( 15 ), 5242–5249. 10.1523/JNEUROSCI.4135-11.2012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After . Annual Review of Neuroscience , 35 ( 1 ), 73–89. 10.1146/annurev-neuro-062111-150525 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Petitmengin C, & Lachaux J-P (2013). Microcognitive science: Bridging experiential and neuronal microdynamics . Frontiers in Human Neuroscience , 7 10.3389/fnhum.2013.00617 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poerio GL, Totterdell P, & Miles E (2013). Mind-wandering and negative mood: Does one thing really lead to another? Consciousness and Cognition , 22 ( 4 ), 1412–1421. 10.1016/j.concog.2013.09.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Posner MI, & Petersen SE (1990). The Attention System of the Human Brain . Annual Review of Neuroscience , 13 ( 1 ), 25–42. 10.1146/annurev.ne.13.030190.000325 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Posner MI, & Rothbart MK (2007). Research on Attention Networks as a Model for the Integration of Psychological Science . Annual Review of Psychology , 58 ( 1 ), 1–23. 10.1146/annurev.psych.58.110405.085516 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ptak R (2012). The Frontoparietal Attention Network of the Human Brain: Action, Saliency, and a Priority Map of the Environment . The Neuroscientist , 18 ( 5 ), 502–515. 10.1177/1073858411409051 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rahl HA, Lindsay EK, Pacilio LE, Brown KW, & Creswell JD (2017). Brief Mindfulness Meditation Training Reduces Mind-Wandering: The Critical Role of Acceptance . Emotion (Washington, D.C.) , 17 ( 2 ), 224–230. 10.1037/emo0000250 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function . Proceedings of the National Academy of Sciences of the United States of America , 98 ( 2 ), 676–682. 10.1073/pnas.98.2.676 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raichle Marcus E. (2015). The Brain’s Default Mode Network . Annual Review of Neuroscience , 38 ( 1 ), 433–447. 10.1146/annurev-neuro-071013-014030 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reichle ED, Reineberg AE, & Schooler JW (2010). Eye Movements During Mindless Reading . Psychological Science , 21 ( 9 ), 1300–1310. 10.1177/0956797610378686 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, & Beasley D (2010). Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice . Journal of Psychosomatic Research , 68 ( 1 ), 29–36. 10.1016/j.jpsychores.2009.03.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning . Trends in Cognitive Sciences , 16 ( 3 ), 147–156. 10.1016/j.tics.2012.01.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ruby FJM, Smallwood J, Engen H, & Singer T (2013). How self-generated thought shapes mood—The relation between mind-wandering and mood depends on the socio-temporal content of thoughts . PloS One , 8 ( 10 ), e77554 10.1371/journal.pone.0077554 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sanger KL, & Dorjee D (2016). Mindfulness training with adolescents enhances metacognition and the inhibition of irrelevant stimuli: Evidence from event-related brain potentials . Trends in Neuroscience and Education , 5 ( 1 ), 1–11. 10.1016/j.tine.2016.01.001 [ CrossRef ] [ Google Scholar ]
- Sauseng P, Klimesch W, Schabus M, & Doppelmayr M (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory . International Journal of Psychophysiology , 57 ( 2 ), 97–103. 10.1016/j.ijpsycho.2005.03.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schooler JW, Mrazek MD, Franklin MS, Baird B, Mooneyham BW, Zedelius C, & Broadway JM (2014). Chapter One - The Middle Way: Finding the Balance between Mindfulness and Mind-Wandering . In Ross BH (Ed.), Psychology of Learning and Motivation (Vol. 60 , pp. 1–33). 10.1016/B978-0-12-800090-8.00001-9 [ CrossRef ] [ Google Scholar ]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control . Journal of Neuroscience , 27 ( 9 ), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Segal ZV, Teasdale JD, & Williams JMG (2004). Mindfulness-Based Cognitive Therapy: Theoretical Rationale and Empirical Status In Mindfulness and acceptance: Expanding the cognitive-behavioral tradition (pp. 45–65). New York, NY, US: Guilford Press. [ Google Scholar ]
- Seli P, Kane MJ, Smallwood J, Schacter DL, Maillet D, Schooler JW, & Smilek D (2018). Mind-Wandering as a Natural Kind: A Family-Resemblances View . Trends in Cognitive Sciences , 22 ( 6 ), 479–490. 10.1016/j.tics.2018.03.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharf RH (2015). Is mindfulness Buddhist? (And why it matters) . Transcultural Psychiatry , 52 ( 4 ), 470–484. 10.1177/1363461514557561 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shaw EE, Schultz AP, Sperling RA, & Hedden T (2015). Functional Connectivity in Multiple Cortical Networks Is Associated with Performance Across Cognitive Domains in Older Adults . Brain Connectivity , 5 ( 8 ), 505–516. 10.1089/brain.2014.0327 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singer JL (1966). Daydreaming: An introduction to the experimental study of inner experience . New York, NY, US: Crown Publishing Group/Random House. [ Google Scholar ]
- Singh NN, Lancioni GE, Wahler RG, Winton ASW, & Singh J (2008). Mindfulness approaches in cognitive behavior therapy . Behavioural and Cognitive Psychotherapy , 36 ( 6 ), 659–666. 10.1017/S1352465808004827 [ CrossRef ] [ Google Scholar ]
- Slagter HA, Davidson RJ, & Lutz A (2011). Mental Training as a Tool in the Neuroscientific Study of Brain and Cognitive Plasticity . Frontiers in Human Neuroscience , 5 10.3389/fnhum.2011.00017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, & Davidson RJ (2008). Theta Phase Synchrony and Conscious Target Perception: Impact of Intensive Mental Training . Journal of Cognitive Neuroscience , 21 ( 8 ), 1536–1549. 10.1162/jocn.2009.21125 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J (2013). Distinguishing how from why the mind wanders: A process–occurrence framework for self-generated mental activity . Psychological Bulletin , 139 ( 3 ), 519–535. 10.1037/a0030010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J, & Andrews-Hanna J (2013). Not all minds that wander are lost: The importance of a balanced perspective on the mind-wandering state . Frontiers in Psychology , 4 , 441 10.3389/fpsyg.2013.00441 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J, Fishman DJ, & Schooler JW (2007). Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance . Psychonomic Bulletin & Review , 14 ( 2 ), 230–236. [ PubMed ] [ Google Scholar ]
- Smallwood JM, Baracaia SF, Lowe M, & Obonsawin M (2003). Task unrelated thought whilst encoding information . Consciousness and Cognition , 12 ( 3 ), 452–484. 10.1016/S1053-8100(03)00018-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J, McSpadden M, & Schooler JW (2007). The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders . Psychonomic Bulletin & Review , 14 ( 3 ), 527–533. [ PubMed ] [ Google Scholar ]
- Smallwood J, & Schooler JW (2006). The restless mind . Psychological Bulletin , 132 ( 6 ), 946–958. 10.1037/0033-2909.132.6.946 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J, & Schooler JW (2015). The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness . Annual Review of Psychology , 66 ( 1 ), 487–518. 10.1146/annurev-psych-010814-015331 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J, Schooler JW, Turk DJ, Cunningham SJ, Burns P, & Macrae CN (2011). Self-reflection and the temporal focus of the wandering mind . Consciousness and Cognition , 20 ( 4 ), 1120–1126. 10.1016/j.concog.2010.12.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Snyder HR, Kaiser RH, Warren SL, & Heller W (2015). Obsessive-Compulsive Disorder Is Associated With Broad Impairments in Executive Function: A Meta-Analysis . Clinical Psychological Science , 3 ( 2 ), 301–330. 10.1177/2167702614534210 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2012). Intrinsic Architecture Underlying the Relations among the Default, Dorsal Attention, and Frontoparietal Control Networks of the Human Brain . Journal of Cognitive Neuroscience , 25 ( 1 ), 74–86. 10.1162/jocn_a_00281 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stawarczyk D, & D’Argembeau A (2015). Neural correlates of personal goal processing during episodic future thinking and mind-wandering: An ALE meta-analysis . Human Brain Mapping , 36 ( 8 ), 2928–2947. 10.1002/hbm.22818 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stawarczyk D, Majerus S, & D’Argembeau A (2013). Concern-induced negative affect is associated with the occurrence and content of mind-wandering . Consciousness and Cognition , 22 ( 2 ), 442–448. 10.1016/j.concog.2013.01.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stawarczyk D, Majerus S, Maquet P, & D’Argembeau A (2011). Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity . PloS One , 6 ( 2 ), e16997 10.1371/journal.pone.0016997 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swick D, & Turken AU (2002). Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex . Proceedings of the National Academy of Sciences , 99 ( 25 ), 16354–16359. 10.1073/pnas.252521499 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sze JA, Gyurak A, Yuan JW, & Levenson RW (2010). Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion , 10 ( 6 ), 803–814. 10.1037/a0020146 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tang Y-Y, Hölzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation . Nature Reviews Neuroscience , 16 ( 4 ), 213–225. 10.1038/nrn3916 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tang Y-Y, & Posner MI (2009). Attention training and attention state training . Trends in Cognitive Sciences , 13 ( 5 ), 222–227. 10.1016/j.tics.2009.01.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tang Y-Y, & Posner MI (2014). Training brain networks and states . Trends in Cognitive Sciences , 18 ( 7 ), 345–350. 10.1016/j.tics.2014.04.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tang Y-Y, Rothbart MK, & Posner MI (2012). Neural correlates of establishing, maintaining, and switching brain states . Trends in Cognitive Sciences , 16 ( 6 ), 330–337. 10.1016/j.tics.2012.05.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, … Creswell JD (2015). Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial . Social Cognitive and Affective Neuroscience , 10 ( 12 ), 1758–1768. 10.1093/scan/nsv066 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, … Beauregard M (2013). Impact of meditation training on the default mode network during a restful state . Social Cognitive and Affective Neuroscience , 8 ( 1 ), 4–14. 10.1093/scan/nsr087 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teper R, & Inzlicht M (2013). Meditation, mindfulness and executive control: The importance of emotional acceptance and brain-based performance monitoring . Social Cognitive and Affective Neuroscience , 8 ( 1 ), 85–92. 10.1093/scan/nss045 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teper R, Segal ZV, & Inzlicht M (2013). Inside the Mindful Mind: How Mindfulness Enhances Emotion Regulation Through Improvements in Executive Control . Current Directions in Psychological Science , 22 ( 6 ), 449–454. 10.1177/0963721413495869 [ CrossRef ] [ Google Scholar ]
- Thompson E (2008). Neurophenomenology and Contemplative Experience . The Oxford Handbook of Religion and Science 10.1093/oxfordhb/9780199543656.003.0015 [ CrossRef ] [ Google Scholar ]
- Todd RM, Cunningham WA, Anderson AK, & Thompson E (2012). Affect-biased attention as emotion regulation . Trends in Cognitive Sciences , 16 ( 7 ), 365–372. 10.1016/j.tics.2012.06.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Travis F, & Shear J (2010). Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions . Consciousness and Cognition , 19 ( 4 ), 1110–1118. 10.1016/j.concog.2010.01.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tulving E (2002). Episodic memory: From mind to brain . Annual Review of Psychology , 53 , 1–25. 10.1146/annurev.psych.53.100901.135114 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tulving E, & Craik FIM (2005). The Oxford Handbook of Memory . Oxford University Press. [ Google Scholar ]
- Unsworth N, & McMillan B (2013). Mind Wandering and Reading Comprehension: Examining the Roles of Working Memory Capacity, Interest, Motivation, and Topic Experience . Journal of Experimental Psychology: Learning, Memory, and Cognition , 39 ( 3 ), 832–842. 10.1037/a0029669 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vago D, & Silbersweig D (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness . Frontiers in Human Neuroscience , 6 10.3389/fnhum.2012.00296 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vago D, & Zeidan F (2016). The brain on silent: Mind wandering, mindful awareness, and states of mental tranquility . Annals of the New York Academy of Sciences , 1373 ( 1 ), 96–113. 10.1111/nyas.13171 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Valentine ER, & Sweet PLG (1999). Meditation and attention: A comparison of the effects of concentrative and mindfulness meditation on sustained attention . Mental Health, Religion & Culture , 2 ( 1 ), 59–70. 10.1080/13674679908406332 [ CrossRef ] [ Google Scholar ]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, … Meyer DE (2018). Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation . Perspectives on Psychological Science , 13 ( 1 ), 36–61. 10.1177/1745691617709589 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van den Hurk PAM, Giommi F, Gielen SC, Speckens AEM, & Barendregt HP (2010). Greater efficiency in attentional processing related to mindfulness meditation . Quarterly Journal of Experimental Psychology , 63 ( 6 ), 1168–1180. 10.1080/17470210903249365 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Lutterveld R, Houlihan SD, Pal P, Sacchet MD, McFarlane-Blake C, Patel PR, … Brewer JA (2017). Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation . NeuroImage , 151 , 117–127. 10.1016/j.neuroimage.2016.02.047 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Veen V, & Carter CS (2002). The anterior cingulate as a conflict monitor: FMRI and ERP studies . Physiology & Behavior , 77 ( 4–5 ), 477–482. [ PubMed ] [ Google Scholar ]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, … Laureys S (2011). Two distinct neuronal networks mediate the awareness of environment and of self . Journal of Cognitive Neuroscience , 23 ( 3 ), 570–578. 10.1162/jocn.2010.21488 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity . Journal of Neurophysiology , 100 ( 6 ), 3328–3342. 10.1152/jn.90355.2008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wahbeh H, Sagher A, Back W, Pundhir P, & Travis F (2018). A Systematic Review of Transcendent States Across Meditation and Contemplative Traditions . EXPLORE , 14 ( 1 ), 19–35. 10.1016/j.explore.2017.07.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wallace BA (1999, February 1). The Buddhist tradition of Samatha: Methods for refining and examining consciousness [Text] Retrieved October 8, 2018, from https://www.ingentaconnect.com/content/imp/jcs/1999/00000006/F0020002/932
- Watkins ER (2008). Constructive and unconstructive repetitive thought . Psychological Bulletin , 134 ( 2 ), 163–206. 10.1037/0033-2909.134.2.163 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, … Kong J (2013). Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: A pilot study . Neuroscience Letters , 556 , 15–19. 10.1016/j.neulet.2013.10.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weng HY, Fox AS, Shackman AJ, Stodola DE, Caldwell JZK, Olson MC, … Davidson RJ (2013). Compassion Training Alters Altruism and Neural Responses to Suffering . Psychological Science , 24 ( 7 ), 1171–1180. 10.1177/0956797612469537 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wenk-Sormaz H (2005). Meditation can reduce habitual responding . Alternative Therapies in Health and Medicine , 11 ( 2 ), 42–58. [ PubMed ] [ Google Scholar ]
- Whitfield-Gabrieli S, & Ford JM (2012). Default Mode Network Activity and Connectivity in Psychopathology . Annual Review of Clinical Psychology , 8 ( 1 ), 49–76. 10.1146/annurev-clinpsy-032511-143049 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whitmarsh S, Barendregt H, Schoffelen J-M, & Jensen O (2014). Metacognitive awareness of covert somatosensory attention corresponds to contralateral alpha power . NeuroImage , 85 , 803–809. 10.1016/j.neuroimage.2013.07.031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yordanova J, Kolev V, & Rothenberger A (2013). Chapter 18—Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder . In Başar E, Başar-Eroĝlu C, Özerdem A, Rossini PM, & Yener GG (Eds.), Supplements to Clinical Neurophysiology (pp. 289–301). 10.1016/B978-0-7020-5307-8.00018-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Young CB, Raz G, Everaerd D, Beckmann CF, Tendolkar I, Hendler T, … Hermans EJ (2017). Dynamic Shifts in Large-Scale Brain Network Balance As a Function of Arousal . Journal of Neuroscience , 37 ( 2 ), 281–290. 10.1523/JNEUROSCI.1759-16.2016 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zanesco AP, King BG, MacLean KA, Jacobs TL, Aichele SR, Wallace BA, … Saron CD (2016). Meditation training influences mind wandering and mindless reading . Psychology of Consciousness: Theory, Research, and Practice , 3 ( 1 ), 12–33. 10.1037/cns0000082 [ CrossRef ] [ Google Scholar ]
- Zedelius CM, & Schooler JW (2015). Mind wandering “Ahas” versus mindful reasoning: Alternative routes to creative solutions . Frontiers in Psychology , 6 10.3389/fpsyg.2015.00834 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zeidan F, Johnson SK, Diamond BJ, David Z, & Goolkasian P (2010). Mindfulness meditation improves cognition: Evidence of brief mental training . Consciousness and Cognition , 19 ( 2 ), 597–605. 10.1016/j.concog.2010.03.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
Why Do Our Minds Wander?
A scientist says mind-wandering or daydreaming help prepare us for the future
Tim Vernimmen, Knowable Magazine
:focal(800x602:801x603)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/a5/8b/a58b6ad5-aabb-49b6-9565-941c9ce047f0/mind-wandering-1200px_web.jpg)
When psychologist Jonathan Smallwood set out to study mind-wandering about 25 years ago, few of his peers thought that was a very good idea. How could one hope to investigate these spontaneous and unpredictable thoughts that crop up when people stop paying attention to their surroundings and the task at hand? Thoughts that couldn’t be linked to any measurable outward behavior?
But Smallwood, now at Queen’s University in Ontario, Canada, forged ahead. He used as his tool a downright tedious computer task that was intended to reproduce the kinds of lapses of attention that cause us to pour milk into someone’s cup when they asked for black coffee. And he started out by asking study participants a few basic questions to gain insight into when and why minds tend to wander, and what subjects they tend to wander toward. After a while, he began to scan participants’ brains as well, to catch a glimpse of what was going on in there during mind-wandering.
Smallwood learned that unhappy minds tend to wander in the past, while happy minds often ponder the future . He also became convinced that wandering among our memories is crucial to help prepare us for what is yet to come. Though some kinds of mind-wandering — such as dwelling on problems that can’t be fixed — may be associated with depression , Smallwood now believes mind-wandering is rarely a waste of time. It is merely our brain trying to get a bit of work done when it is under the impression that there isn’t much else going on.
Smallwood, who coauthored an influential 2015 overview of mind-wandering research in the Annual Review of Psychology, is the first to admit that many questions remain to be answered.
This conversation has been edited for length and clarity.
Is mind-wandering the same thing as daydreaming, or would you say those are different?
I think it’s a similar process used in a different context. When you’re on holiday, and you’ve got lots of free time, you might say you’re daydreaming about what you’d like to do next. But when you’re under pressure to perform, you’d experience the same thoughts as mind-wandering.
I think it is more helpful to talk about the underlying processes: spontaneous thought, or the decoupling of attention from perception, which is what happens when our thoughts separate from our perception of the environment. Both these processes take place during mind-wandering and daydreaming.
It often takes us a while to catch ourselves mind-wandering. How can you catch it to study it in other people?
In the beginning, we gave people experimental tasks that were really boring, so that mind-wandering would happen a lot. We would just ask from time to time, “Are you mind-wandering?” while recording the brain’s activity in an fMRI scanner.
But what I’ve realized, after doing studies like that for a long time, is that if we want to know how thinking works in the real world, where people are doing things like watching TV or going for a run, most of the data we have are never going to tell us very much.
So we are now trying to study these situations . And instead of doing experiments where we just ask, “Are you mind-wandering?” we are now asking people a lot of different questions, like: “Are your thoughts detailed? Are they positive? Are they distracting you?”
How and why did you decide to study mind-wandering?
I started studying mind-wandering at the start of my career, when I was young and naive.
I didn’t really understand at the time why nobody was studying it. Psychology was focused on measurable, outward behavior then. I thought to myself: That’s not what I want to understand about my thoughts. What I want to know is: Why do they come, where do they come from, and why do they persist even if they interfere with attention to the here and now?
Around the same time, brain imaging techniques were developing, and they were telling neuroscientists that something happens in the brain even when it isn’t occupied with a behavioral task. Large regions of the brain, now called the default mode network , did the opposite: If you gave people a task, the activity in these areas went down.
When scientists made this link between brain activity and mind-wandering, it became fashionable. I’ve been very lucky, because I hadn’t anticipated any of that when I started my PhD, at the University of Strathclyde in Glasgow. But I’ve seen it all pan out.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/4c/58/4c5891e3-0206-47df-9255-a8d5e196de9f/g-default-mode-network-alt_web.jpg)
Would you say, then, that mind-wandering is the default mode for our brains?
It turns out to be more complicated than that. Initially, researchers were very sure that the default mode network rarely increased its activity during tasks. But these tasks were all externally focused — they involved doing something in the outside world. When researchers later asked people to do a task that doesn’t require them to interact with their environment — like think about the future — that activated the default mode network as well.
More recently, we have identified much simpler tasks that also activate the default mode network. If you let people watch a series of shapes like triangles or squares on a screen, and every so often you surprise them and ask something — like, “In the last trial, which side was the triangle on?”— regions within the default mode network increase activity when they’re making that decision . That’s a challenging observation if you think the default mode network is just a mind-wandering system.
But what both situations have in common is the person is using information from memory. I now think the default mode network is necessary for any thinking based on information from memory — and that includes mind-wandering.
Would it be possible to demonstrate that this is indeed the case?
In a recent study, instead of asking people whether they were paying attention, we went one step further . People were in a scanner reading short factual sentences on a screen. Occasionally, we’d show them a prompt that said, “Remember,” followed by an item from a list of things from their past that they’d provided earlier. So then, instead of reading, they’d remember the thing we showed them. We could cause them to remember.
What we find is that the brain scans in this experiment look remarkably similar to mind-wandering. That is important: It gives us more control over the pattern of thinking than when it occurs spontaneously, like in naturally occurring mind-wandering. Of course, that is a weakness as well, because it’s not spontaneous. But we’ve already done lots of spontaneous studies.
When we make people remember things from the list, we recapitulate quite a lot of what we saw in spontaneous mind-wandering. This suggests that at least some of the activity we see when minds wander is indeed associated with the retrieval of memories. We now think the decoupling between attention and perception happens because people are remembering.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/f4/73/f473de94-c653-4155-be90-aa06c5d9a827/g-brain-regions-mind-wandering-alt_web.jpg)
Have you asked people what their minds are wandering toward?
The past and future seem to really dominate people’s thinking . I think things like mind-wandering are attempts by the brain to make sense of what has happened, so that we can behave better in the future. I think this type of thinking is a really ingrained part of how our species has conquered the world. Almost nothing we’re doing at any moment in time can be pinpointed as only mattering then.
That’s a defining difference. By that, I don’t mean that other animals can’t imagine the future, but that our world is built upon our ability to do so, and to learn from the past to build a better future. I think animals that focused only on the present were outcompeted by others that remembered things from the past and could focus on future goals, for millions of years — until you got humans, a species that’s obsessed with taking things that happened and using them to gain added value for future behavior.
People are also, very often, mind-wandering about social situations . This makes sense, because we have to work with other people to achieve almost all of our goals, and other people are much more unpredictable than the Sun rising in the morning.
Though it is clearly useful, isn’t it also very depressing to keep returning to issues from the past?
It certainly can be. We have found that mind-wandering about the past tends to be associated with negative mood.
Let me give you an example of what I think may be happening. For a scientist like me, coming up with creative solutions to scientific problems through mind-wandering is very rewarding. But you can imagine that if my situation changes and I end up with a set of problems I can’t fix, the habit of going over the past may become difficult to break. My brain will keep activating the problem-solving system, even if it can’t do anything to fix the problem, because now my problems are things like getting divorced and my partner doesn’t want any more to do with me. If such a thing happens and all I’ve got is an imaginative problem-solving system, it’s not going to help me, it’s just going to be upsetting. I just have to let it go.
That’s where I think mindfulness could be useful, because the idea of mindfulness is to bring your attention to the moment. So if I’d be more mindful, I’d be going into problem-solving mode less often.
If you spend long enough practicing being in the moment, maybe that becomes a habit. It’s about being able to control your mind-wandering. Cognitive behavioral therapy for depression, which aims to help people change how they think and behave, is another way to reduce harmful mind-wandering.
Nowadays, it seems that many of the idle moments in which our minds would previously have wandered are now spent scrolling our phones. How do you think that might change how our brain functions?
The interesting thing about social media and mind-wandering, I think, is that they may have similar motivations. Mind-wandering is very social. In our studies , we’re locking people in small booths and making them do these tasks and they keep coming out and saying, “I’m thinking about my friends.” That’s telling us that keeping up with others is very important to people.
Social groups are so important to us as a species that we spend most of our time trying to anticipate what others are going to do, and I think social media is filling part of the gap that mind-wandering is trying to fill. It’s like mainlining social information: You can try to imagine what your friend is doing, or you can just find out online. Though, of course, there is an important difference: When you’re mind-wandering, you’re ordering your own thoughts. Scrolling social media is more passive.
Could there be a way for us to suppress mind-wandering in situations where it might be dangerous?
Mind-wandering can be a benefit and a curse, but I wouldn’t be confident that we know yet when it would be a good idea to stop it. In our studies at the moment, we are trying to map how people think across a range of different types of tasks. We hope this approach will help us identify when mind-wandering is likely to be useful or not — and when we should try to control it and when we shouldn’t.
For example, in our studies, people who are more intelligent don’t mind wander so often when the task is hard but can do it more when tasks are easy . It is possible that they are using the idle time when the external world is not demanding their attention to think about other important matters. This highlights the uncertainty about whether mind wandering is always a bad thing, because this sort of result implies it is likely to be useful under some circumstances.
This map — of how people think in different situations — has become very important in our research. This is the work I’m going to focus on now, probably for the rest of my career.

Get the latest Science stories in your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 22 September 2016
Mind-wandering as spontaneous thought: a dynamic framework
- Kalina Christoff 1 , 2 ,
- Zachary C. Irving 3 ,
- Kieran C. R. Fox 1 ,
- R. Nathan Spreng 4 , 5 &
- Jessica R. Andrews-Hanna 6
Nature Reviews Neuroscience volume 17 , pages 718–731 ( 2016 ) Cite this article
25k Accesses
677 Citations
493 Altmetric
Metrics details
- Cognitive control
- Schizophrenia
In the past 15 years, mind-wandering has become a prominent topic in cognitive neuroscience and psychology. Whereas mind-wandering has come to be predominantly defined as task-unrelated and/or stimulus-unrelated thought, we argue that this content-based definition fails to capture the defining quality of mind-wandering: the relatively free and spontaneous arising of mental states as the mind wanders.
We define spontaneous thought as a mental state, or a sequence of mental states, that arises relatively freely due to an absence of strong constraints on the contents of each state and on the transitions from one mental state to another. We propose that there are two general ways in which the content of mental states, and the transitions between them, can be constrained.
Deliberate and automatic constraints serve to limit the contents of thought and how these contents change over time. Deliberate constraints are implemented through cognitive control, whereas automatic constraints can be considered as a family of mechanisms that operate outside of cognitive control, including sensory or affective salience.
Within our framework, mind-wandering can be defined as a special case of spontaneous thought that tends to be more deliberately constrained than dreaming, but less deliberately constrained than creative thinking and goal-directed thought. In addition, mind-wandering can be clearly distinguished from rumination and other types of thought that are marked by a high degree of automatic constraints, such as obsessive thought.
In general, deliberate constraints are minimal during dreaming, tend to increase somewhat during mind-wandering, increase further during creative thinking and are strongest during goal-directed thought. There is a range of low-to-medium level of automatic constraints that can occur during dreaming, mind-wandering and creative thinking, but thought ceases to be spontaneous at the strongest levels of automatic constraint, such as during rumination or obsessive thought.
We propose a neural model of the interactions among sources of variability, automatic constraints and deliberate constraints on thought: the default network (DN) subsystem centred around the medial temporal lobe (MTL) (DN MTL ) and sensorimotor areas can act as sources of variability; the salience networks, the dorsal attention network (DAN) and the core DN subsystem (DN CORE ) can exert automatic constraints on the output of the DN MTL and sensorimotor areas, thus limiting the variability of thought; and the frontoparietal control network can exert deliberate constraints on thought by flexibly coupling with the DN CORE , the DAN or the salience networks, thus reinforcing or reducing the automatic constraints being exerted by the DN CORE , the DAN or the salience networks.
Most research on mind-wandering has characterized it as a mental state with contents that are task unrelated or stimulus independent. However, the dynamics of mind-wandering — how mental states change over time — have remained largely neglected. Here, we introduce a dynamic framework for understanding mind-wandering and its relationship to the recruitment of large-scale brain networks. We propose that mind-wandering is best understood as a member of a family of spontaneous-thought phenomena that also includes creative thought and dreaming. This dynamic framework can shed new light on mental disorders that are marked by alterations in spontaneous thought, including depression, anxiety and attention deficit hyperactivity disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
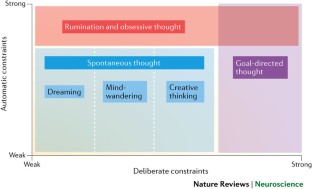
Similar content being viewed by others
Recent advances in the neuroscience of spontaneous and off-task thought: implications for mental health
Aaron Kucyi, Julia W. Y. Kam, … Susan Whitfield-Gabrieli
Introspective and Neurophysiological Measures of Mind Wandering in Schizophrenia
S. Iglesias-Parro, M. F. Soriano, … A. J. Ibáñez-Molina
Prediction of stimulus-independent and task-unrelated thought from functional brain networks
Aaron Kucyi, Michael Esterman, … Susan Whitfield-Gabrieli
James, W. The Principles of Psychology (Henry Holt and Company, 1890).
Google Scholar
Callard, F., Smallwood, J., Golchert, J. & Margulies, D. S. The era of the wandering mind? Twenty-first century research on self-generated mental activity. Front. Psychol. 4 , 891 (2013).
PubMed PubMed Central Google Scholar
Andreasen, N. C. et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry 152 , 1576–1585 (1995).
CAS PubMed Google Scholar
Binder, J. R., Frost, J. A. & Hammeke, T. A. Conceptual processing during the conscious resting state: a functional MRI study. J. Cogn. Neurosci. 11 , 80–93 (1999).
Stark, C. E. & Squire, L. R. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc. Natl Acad. Sci. USA 98 , 12760–12766 (2001).
CAS PubMed PubMed Central Google Scholar
Christoff, K. & Gabrieli, J. D. E. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28 , 168–186 (2000).
Shulman, G. L. et al. Common blood flow changes across visual tasks: II. Decreases cerebral cortex. J. Cogn. Neurosci. 9 , 648–663 (1997). This meta-analysis provides convincing evidence that a set of specific brain regions, which later became known as the default mode network, becomes consistently activated during rest.
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98 , 676–682 (2001). This highly influential theoretical paper coined the term 'default mode' to refer to cognitive and neural processes that occur in the absence of external task demands.
Singer, J. L. Daydreaming: An Introduction to the Experimental Study of Inner Experience (Random House, 1966).
Antrobus, J. S. Information theory and stimulus-independent thought. Br. J. Psychol. 59 , 423–430 (1968).
Antrobus, J. S., Singer, J. L., Goldstein, S. & Fortgang, M. Mind wandering and cognitive structure. Trans. NY Acad. Sci. 32 , 242–252 (1970).
CAS Google Scholar
Filler, M. S. & Giambra, L. M. Daydreaming as a function of cueing and task difficulty. Percept. Mot. Skills 37 , 503–509 (1973).
Giambra, L. M. Adult male daydreaming across the life span: a replication, further analyses, and tentative norms based upon retrospective reports. Int. J. Aging Hum. Dev. 8 , 197–228 (1977).
PubMed Google Scholar
Giambra, L. M. Sex differences in daydreaming and related mental activity from the late teens to the early nineties. Int. J. Aging Hum. Dev. 10 , 1–34 (1979).
Klinger, E. & Cox, W. M. Dimensions of thought flow in everyday life. Imagin. Cogn. Pers. 7 , 105–128 (1987). This is probably the earliest experience sampling study of mind-wandering in daily life, revealing that adults spend approximately one-third of their waking life engaged in undirected thinking.
Giambra, L. M. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol. Aging 4 , 136–143 (1989).
Teasdale, J. D., Proctor, L., Lloyd, C. A. & Baddeley, A. D. Working memory and stimulus-independent thought: effects of memory load and presentation rate. Eur. J. Cogn. Psychol. 5 , 417–433 (1993).
Giambra, L. M. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Conscious. Cogn. 4 , 1–21 (1995).
Klinger, E. Structure and Functions of Fantasy (John Wiley & Sons, 1971). This pioneering book summarizes the early empirical research on daydreaming and introduces important theoretical hypotheses, including the idea that task-unrelated thoughts are often about 'current concerns'.
Smallwood, J. & Schooler, J. W. The restless mind. Psychol. Bull. 132 , 946–958 (2006). This paper put mind-wandering in the forefront of psychological research, advancing the influential hypothesis that executive resources support mind-wandering.
Killingsworth, M. A. & Gilbert, D. T. A wandering mind is an unhappy mind. Science 330 , 932 (2010).
Mason, M. F. et al. Wandering minds: the default network and stimulus-independent thought. Science 315 , 393–395 (2007). This influential paper brought mind-wandering to the forefront of neuroscientific research, arguing for a link between DN recruitment and stimulus-independent thought.
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R. & Schooler, J. W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl Acad. Sci. USA 106 , 8719–8724 (2009). This paper is the first to use online experience sampling to examine the neural correlates of mind-wandering and the first to find joint activation of the DN and executive network during this phenomenon.
Callard, F., Smallwood, J. & Margulies, D. S. Default positions: how neuroscience's historical legacy has hampered investigation of the resting mind. Front. Psychol. 3 , 321 (2012).
Smallwood, J. & Schooler, J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol. 66 , 487–518 (2015). This comprehensive review synthesizes the recent research characterizing mind-wandering as task-unrelated and/or stimulus-independent thought.
Christoff, K. Undirected thought: neural determinants and correlates. Brain Res. 1428 , 51–59 (2012). This review disambiguates between different definitions of spontaneous thought and mind-wandering, and it argues that current definitions do not capture the dynamics of thought.
Irving, Z. C. Mind-wandering is unguided attention: accounting for the 'purposeful' wanderer. Philos. Stud. 173 , 547–571 (2016). This is one of the first philosophical theories of mind-wandering; this paper defines mind-wandering as unguided attention to explain why its dynamics contrast with automatically and deliberately guided forms of attention such as rumination and goal-directed thinking.
Carruthers, P. The Centered Mind: What the Science of Working Memory Shows Us About the Nature of Human Thought (Oxford Univ. Press, 2015).
Simpson, J. A. The Oxford English Dictionary (Clarendon Press, 1989).
Kane, M. J. et al. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol. Sci. 18 , 614–621 (2007). This study of mind-wandering in everyday life is one of the most important investigations into the complex relationship between mind-wandering and executive control.
Baird, B., Smallwood, J. & Schooler, J. W. Back to the future: autobiographical planning and the functionality of mind-wandering. Conscious. Cogn. 20 , 1604–1611 (2011).
Morsella, E., Ben-Zeev, A., Lanska, M. & Bargh, J. A. The spontaneous thoughts of the night: how future tasks breed intrusive cognitions. Social Cogn. 28 , 641–650 (2010).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24 , 167–202 (2001).
Miller, E. K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 1 , 59–65 (2000).
Markovic, J., Anderson, A. K. & Todd, R. M. Tuning to the significant: neural and genetic processes underlying affective enhancement of visual perception and memory. Behav. Brain Res. 259 , 229–241 (2014).
Todd, R. M., Cunningham, W. A., Anderson, A. K. & Thompson, E. Affect-biased attention as emotion regulation. Trends Cogn. Sci. 16 , 365–372 (2012).
Pessoa, L. The Cognitive-Emotional Brain: From Interactions to Integration (MIT Press, 2013).
Jonides, J. & Yantis, S. Uniqueness of abrupt visual onset in capturing attention. Percept. Psychophys. 43 , 346–354 (1988).
Christoff, K., Gordon, A. M. & Smith, R. in Neuroscience of Decision Making (eds Vartanian, O. & Mandel, D. R.) 259–284 (Psychology Press, 2011).
Stawarczyk, D., Majerus, S., Maj, M., Van der Linden, M. & D'Argembeau, A. Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychol. (Amst.) 136 , 370–381 (2011).
Spreng, R. N., Mar, R. A. & Kim, A. S. N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21 , 489–510 (2009). This paper provides some of the first quantitative evidence that the DN is associated with multiple cognitive functions.
Andrews-Hanna, J. R. The brain's default network and its adaptive role in internal mentation. Neuroscientist 18 , 251–270 (2012). This recent review describes a large-scale functional meta-analysis on the cognitive functions, functional subdivisions and clinical dysfunction of the DN.
Buckner, R. L. & Carroll, D. C. Self-projection and the brain. Trends Cogn. Sci. 11 , 49–57 (2007).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124 , 1–38 (2008). This comprehensive review bridges across neuroscience, psychology and clinical research, and introduces a prominent hypothesis — the 'internal mentation hypothesis' — that the DN has an important role in spontaneous and directed forms of internal mentation.
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-anatomic fractionation of the brain's default network. Neuron 65 , 550–562 (2010).
Schacter, D. L., Addis, D. R. & Buckner, R. L. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 8 , 657–661 (2007).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. NY Acad. Sci. 1316 , 29–52 (2014).
Corbetta, M., Patel, G. & Shulman, G. L. The reorienting system of the human brain: from environment to theory of mind. Neuron 58 , 306–324 (2008). This paper outlines an influential theoretical framework that extends an earlier model by Corbetta and Shulman that drew a crucial distinction between the DAN and VAN.
Vanhaudenhuyse, A. et al. Two distinct neuronal networks mediate the awareness of environment and of self. J. Cogn. Neurosci. 23 , 570–578 (2011).
Smallwood, J. Distinguishing how from why the mind wanders: a process–occurrence framework for self-generated mental activity. Psychol. Bull. 139 , 519–535 (2013). This theoretical paper presents an important distinction between the events that determine when an experience initially occurs from the processes that sustain an experience over time.
Toro, R., Fox, P. T. & Paus, T. Functional coactivation map of the human brain. Cereb. Cortex 18 , 2553–2559 (2008).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102 , 9673–9678 (2005). This paper provides a unique insight into the functional antagonism between the default and dorsal attention systems.
CAS PubMed Central PubMed Google Scholar
Keller, C. J. et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J. Neurosci. 33 , 6333–6342 (2013).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27 , 2349–2356 (2007). This paper is the first to name the salience network and characterize its functional neuroanatomy.
Kucyi, A., Hodaie, M. & Davis, K. D. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J. Neurophysiol. 108 , 3382–3392 (2012).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72 , 665–678 (2011).
Cole, M. W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16 , 1348–1355 (2013).
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E. & Buckner, R. L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100 , 3328–3342 (2008).
Niendam, T. A. et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 12 , 241–268 (2012).
Spreng, R. N., Stevens, W. D., Chamberlain, J. P., Gilmore, A. W. & Schacter, D. L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53 , 303–317 (2010). This paper demonstrates how the DN couples with the FPCN for personally salient, goal-directed information processing.
Dixon, M. L., Fox, K. C. R. & Christoff, K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia 62 , 321–330 (2014).
Dosenbach, N. U. F. et al. A core system for the implementation of task sets. Neuron 50 , 799–812 (2006).
Dosenbach, N. U. F. et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl Acad. Sci. USA 104 , 11073–11078 (2007).
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L. & Petersen, S. E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 12 , 99–105 (2008).
Yeo, B. T. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106 , 1125–1165 (2011). This seminal paper uses resting-state functional connectivity and clustering approaches in 1,000 individuals to parcellate the brain into seven canonical large-scale networks.
Najafi, M., McMenamin, B. W., Simon, J. Z. & Pessoa, L. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage 135 , 92–106 (2016).
McGuire, P. K., Paulesu, E., Frackowiak, R. S. & Frith, C. D. Brain activity during stimulus independent thought. Neuroreport 7 , 2095–2099 (1996).
McKiernan, K. A., D'Angelo, B. R., Kaufman, J. N. & Binder, J. R. Interrupting the 'stream of consciousness': an fMRI investigation. Neuroimage 29 , 1185–1191 (2006).
Gilbert, S. J., Dumontheil, I., Simons, J. S., Frith, C. D. & Burgess, P. W. Comment on 'wandering minds: the default network and stimulus-independent thought'. Science 317 , 43b (2007).
Stawarczyk, D., Majerus, S., Maquet, P. & D'Argembeau, A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS ONE 6 , e16997 (2011).
Fox, K. C. R., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R. & Christoff, K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111 , 611–621 (2015). This paper presents the first quantitative meta-analysis of neuroimaging studies on task-unrelated and/or stimulus-independent thought, revealing the involvement of the DN and other large-scale networks that were not traditionally thought to play a part in mind-wandering.
Ingvar, D. H. 'Hyperfrontal' distribution of the cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol. Scand. 60 , 12–25 (1979). This paper by David Ingvar, a pioneer of human neuroimaging, provides the original observations that a resting brain is an active one and highlights the finding that prefrontal executive regions are active even at rest.
Christoff, K., Ream, J. M. & Gabrieli, J. D. E. Neural basis of spontaneous thought processes. Cortex 40 , 623–630 (2004).
D'Argembeau, A. et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage 25 , 616–624 (2005).
Spiers, H. J. & Maguire, E. A. Spontaneous mentalizing during an interactive real world task: an fMRI study. Neuropsychologia 44 , 1674–1682 (2006).
Wang, K. et al. Offline memory reprocessing: involvement of the brain's default network in spontaneous thought processes. PLoS ONE 4 , e4867 (2009).
Dumontheil, I., Gilbert, S. J., Frith, C. D. & Burgess, P. W. Recruitment of lateral rostral prefrontal cortex in spontaneous and task-related thoughts. Q. J. Exp. Psychol. 63 , 1740–1756 (2010).
Posner, M. I. & Rothbart, M. K. Attention, self-regulation and consciousness. Phil. Trans. R. Soc. Lond. B 353 , 1915–1927 (1998).
Duncan, J. & Owen, A. M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23 , 475–483 (2000).
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. Conflict monitoring and cognitive control. Psychol. Rev. 108 , 624–652 (2001).
Banich, M. T. Executive function: the search for an integrated account. Curr. Direct. Psychol. Sci. 18 , 89–94 (2009).
Prado, J., Chadha, A. & Booth, J. R. The brain network for deductive reasoning: a quantitative meta-analysis of 28 neuroimaging studies. J. Cogn. Neurosci. 23 , 3483–3497 (2011).
McVay, J. C. & Kane, M. J. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychol. Bull. 136 , 188–197 (2010). This paper presents the theoretically influential control failure hypothesis, which is opposed to the thesis that executive function supports mind-wandering.
Kane, M. J. & McVay, J. C. What mind wandering reveals about executive-control abilities and failures. Curr. Direct. Psychol. Sci. 21 , 348–354 (2012).
Levinson, D. B., Smallwood, J. & Davidson, R. J. The persistence of thought: evidence for a role of working memory in the maintenance of task-unrelated thinking. Psychol. Sci. 23 , 375–380 (2012).
Salthouse, T. A., Fristoe, N., McGuthry, K. E. & Hambrick, D. Z. Relation of task switching to speed, age, and fluid intelligence. Psychol. Aging 13 , 445–461 (1998).
Maillet, D. & Schacter, D. L. From mind wandering to involuntary retrieval: age-related differences in spontaneous cognitive processes. Neuropsychologia 80 , 142–156 (2016).
Axelrod, V., Rees, G., Lavidor, M. & Bar, M. Increasing propensity to mind-wander with transcranial direct current stimulation. Proc. Natl Acad. Sci. USA 112 , 3314–3319 (2015).
Schooler, J. W. et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn. Sci. 15 , 319–326 (2011).
Smallwood, J., Beach, E. & Schooler, J. W. Going AWOL in the brain: mind wandering reduces cortical analysis of external events. J. Cogn. Neurosci. 20 , 458–469 (2008).
Kam, J. W. Y. et al. Slow fluctuations in attentional control of sensory cortex. J. Cogn. Neurosci. 23 , 460–470 (2011).
Gelbard-Sagiv, H., Mukamel, R., Harel, M., Malach, R. & Fried, I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science 322 , 96–101 (2008). This pioneering study aimed to identify the neural origins of spontaneously recalled memories, finding strong evidence for the initial generation in the MTL.
Ellamil, M. et al. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage 136 , 186–196 (2016). This study is the first to reveal a sequential recruitment of the DN MTL , DN CORE , and FPCN immediately before, during and subsequent to the onset of spontaneous thoughts.
Andrews-Hanna, J. R., Reidler, J. S., Huang, C. & Buckner, R. L. Evidence for the default network's role in spontaneous cognition. J. Neurophysiol. 104 , 322–335 (2010).
Kucyi, A. & Davis, K. D. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 100 , 471–480 (2014).
Foster, D. J. & Wilson, M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440 , 680–683 (2006).
Karlsson, M. P. & Frank, L. M. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12 , 913–918 (2009).
Carr, M. F., Jadhav, S. P. & Frank, L. M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14 , 147–153 (2011).
Dragoi, G. & Tonegawa, S. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl Acad. Sci. USA 110 , 9100–9105 (2013).
Dragoi, G. & Tonegawa, S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469 , 397–401 (2011).
Ólafsdóttir, H. F., Barry, C., Saleem, A. B. & Hassabis, D. Hippocampal place cells construct reward related sequences through unexplored space. eLife 4 , e06063 (2015).
Stark, C. E. L. & Clark, R. E. The medial temporal lobe. Annu. Rev. Neurosci. 27 , 279–306 (2004).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67 , 105–134 (2016).
Romero, K. & Moscovitch, M. Episodic memory and event construction in aging and amnesia. J. Mem. Lang. 67 , 270–284 (2012).
Hassabis, D., Kumaran, D. & Maguire, E. A. Using imagination to understand the neural basis of episodic memory. J. Neurosci. 27 , 14365–14374 (2007).
Buckner, R. L. The role of the hippocampus in prediction and imagination. Annu. Rev. Psychol. 61 , 27–48 (2010).
Hassabis, D. & Maguire, E. A. The construction system of the brain. Phil. Trans. R. Soc. B 364 , 1263–1271 (2009).
Schacter, D. L. et al. The future of memory: remembering, imagining, and the brain. Neuron 76 , 677–694 (2012).
Schacter, D. L., Addis, D. R. & Buckner, R. L. Episodic simulation of future events: concepts, data, and applications. Ann. NY Acad. Sci. 1124 , 39–60 (2008).
Moscovitch, M. Memory and working-with-memory: a component process model based on modules and central systems. J. Cogn. Neurosci. 4 , 257–267 (1992). This paper introduces the influential component process model of memory.
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100 , 147–154 (1986).
Moscovitch, M. The hippocampus as a “stupid,” domain-specific module: implications for theories of recent and remote memory, and of imagination. Can. J. Exp. Psychol. 62 , 62–79 (2008).
Bar, M., Aminoff, E., Mason, M. & Fenske, M. The units of thought. Hippocampus 17 , 420–428 (2007). This paper introduces a novel hypothesis on the associative processes underlying a train of thoughts, originating in the MTL.
Aminoff, E. M., Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17 , 379–390 (2013).
Christoff, K., Keramatian, K., Gordon, A. M., Smith, R. & Mädler, B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 1286 , 94–105 (2009).
Dixon, M. L., Fox, K. C. R. & Christoff, K. Evidence for rostro-caudal functional organization in multiple brain areas related to goal-directed behavior. Brain Res. 1572 , 26–39 (2014).
McCaig, R. G., Dixon, M., Keramatian, K., Liu, I. & Christoff, K. Improved modulation of rostrolateral prefrontal cortex using real-time fMRI training and meta-cognitive awareness. Neuroimage 55 , 1298–1305 (2011).
Dixon, M. L. & Christoff, K. The decision to engage cognitive control is driven by expected reward-value: neural and behavioral evidence. PLoS ONE 7 , e51637 (2012).
Yin, H. H. & Knowlton, B. J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7 , 464–476 (2006).
Burguière. E., Monteiro, P., Mallet, L., Feng, G. & Graybiel, A. M. Striatal circuits, habits, and implications for obsessive–compulsive disorder. Curr. Opin. Neurobiol. 30 , 59–65 (2015).
Mathews, A. & MacLeod, C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 1 , 167–195 (2005).
Gotlib, I. H. & Joormann, J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 6 , 285–312 (2010).
Nolen-Hoeksema, S., Wisco, B. E. & Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci. 3 , 400–424 (2008).
Watkins, E. R. Constructive and unconstructive repetitive thought. Psychol. Bull. 134 , 163–206 (2008). This comprehensive review and theory article links the psychological literature on task-unrelated or stimulus-independent thought to the clinical literature on rumination and other forms of repetitive thought, proposing multiple factors that govern whether repetitive thought is constructive or unconstructive.
Giambra, L. M. & Traynor, T. D. Depression and daydreaming; an analysis based on self-ratings. J. Clin. Psychol. 34 , 14–25 (1978).
Larsen, R. J. & Cowan, G. S. Internal focus of attention and depression: a study of daily experience. Motiv. Emot. 12 , 237–249 (1988).
Whitfield-Gabrieli, S. & Ford, J. M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8 , 49–76 (2012).
Anticevic, A. et al. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 16 , 584–592 (2012).
Hamilton, J. P. et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry 169 , 693–703 (2012).
Kaiser, R. H. et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc. Cogn. Affect. Neurosci. 10 , 654–663 (2015).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry 72 , 603–637 (2015). This meta-analysis of resting-state functional connectivity studies in major depressive disorder provides quantitative support for functional-network imbalances, which reflect heightened internally focused thought in this disorder.
Kaiser, R. H. et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology 41 , 1822–1830 (2015).
Spinhoven, P., Drost, J., van Hemert, B. & Penninx, B. W. Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. J. Anxiety Disord. 33 , 45–52 (2015).
Borkovec, T. D., Ray, W. J. & Stober, J. Worry: a cognitive phenomenon intimately linked to affective, physiological, and interpersonal behavioral processes. Cognit. Ther. Res. 22 , 561–576 (1998).
Oathes, D. J., Patenaude, B., Schatzberg, A. F. & Etkin, A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol. Psychiatry 77 , 385–393 (2015).
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J. & van IJzendoorn, M. H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 133 , 1–24 (2007).
Williams, J., Watts, F. N., MacLeod, C. & Mathews, A. Cognitive Psychology and Emotional Disorders (John Wiley & Sons, 1997).
Etkin, A., Prater, K. E., Schatzberg, A. F., Menon, V. & Greicius, M. D. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry 66 , 1361–1372 (2009).
Ipser, J. C., Singh, L. & Stein, D. J. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci. 67 , 311–322 (2013).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013).
Boonstra, A. M., Oosterlaan, J., Sergeant, J. A. & Buitelaar, J. K. Executive functioning in adult ADHD: a meta-analytic review. Psychol. Med. 35 , 1097–1108 (2005).
Willcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V. & Pennington, B. F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry 57 , 1336–1346 (2005).
Kofler, M. J. et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin. Psychol. Rev. 33 , 795–811 (2013).
Shaw, G. A. & Giambra, L. Task unrelated thoughts of college students diagnosed as hyperactive in childhood. Dev. Neuropsychol. 9 , 17–30 (1993).
Franklin, M. S. et al. Tracking distraction: the relationship between mind-wandering, meta-awareness, and ADHD symptomatology. J. Atten. Disord. http://dx.doi.org/10.1177/1087054714543494 (2014).
De La Fuente, A., Xia, S., Branch, C. & Li, X. A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front. Hum. Neurosci. 7 , 192 (2013).
Castellanos, F. X. & Proal, E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn. Sci. 16 , 17–26 (2012).
Hart, H., Radua, J., Mataix-Cols, D. & Rubia, K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 36 , 2248–2256 (2012).
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D. & Rubia, K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder. JAMA Psychiatry 70 , 185–198 (2013).
Fassbender, C. et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 1273 , 114–128 (2009).
Cortese, S. et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry 169 , 1038–1055 (2012).
Castellanos, F. X. et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 63 , 332–337 (2008).
Uddin, L. Q. et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods 169 , 249–254 (2008).
Tomasi, D. & Volkow, N. D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry 71 , 443–450 (2012).
Mattfeld, A. T. et al. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain 137 , 2423–2428 (2014).
Sun, L. et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res. 201 , 120–127 (2012).
McCarthy, H. et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry 70 , 1329–1337 (2013).
Fair, D. A. et al. The maturing architecture of the brain's default network. Proc. Natl Acad. Sci. USA 105 , 4028–4032 (2008).
Sripada, C. et al. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 35 , 4693–4705 (2014). By analysing data from more than 750 participants, this paper links childhood ADHD to abnormal resting-state functional connectivity involving the DN.
Fair, D. A. et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry 68 , 1084–1091 (2010).
Anderson, A. et al. Non-negative matrix factorization of multimodal MRI, fMRI and phenotypic data reveals differential changes in default mode subnetworks in ADHD. Neuroimage 102 , 207–219 (2014).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59 , 2142–2154 (2012).
Van Dijk, K. R. A., Sabuncu, M. R. & Buckner, R. L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59 , 431–438 (2012).
Sonuga-Barke, E. J. S. & Castellanos, F. X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 31 , 977–986 (2007).
Kerns, J. G. & Berenbaum, H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J. Abnorm. Psychol. 111 , 211–224 (2002).
Videbeck, S. L. Psychiatric Mental Health Nursing (Lippincott Williams & Wilkins, 2006).
Hales, R. E., Yudofsky, S. C. & Roberts, L. W. The American Psychiatric Publishing Textbook of Psychiatry 6th edn (American Psychiatric Publishing, 2014).
Haijma, S. V. et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 39 , 1129–1138 (2013).
Glahn, D. C. et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry 64 , 774–781 (2008).
Fornito, A., Yücel, M., Patti, J., Wood, S. J. & Pantelis, C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr. Res. 108 , 104–113 (2009).
Ellison-Wright, I. & Bullmore, E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 117 , 1–12 (2010).
Vita, A., De Peri, L., Deste, G., Barlati, S. & Sacchetti, E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry 78 , 403–412 (2015).
Cole, M. W., Anticevic, A., Repovs, G. & Barch, D. Variable global dysconnectivity and individual differences in schizophrenia. Biol. Psychiatry 70 , 43–50 (2011).
Argyelan, M. et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr. Bull. 40 , 100–110 (2014).
Cole, M. W., Yarkoni, T., Repovs, G., Anticevic, A. & Braver, T. S. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci. 32 , 8988–8999 (2012).
Baker, J. T. et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry 71 , 109–110 (2014).
Karbasforoushan, H. & Woodward, N. D. Resting-state networks in schizophrenia. Curr. Top. Med. Chem. 12 , 2404–2414 (2013).
Jafri, M. J., Pearlson, G. D., Stevens, M. & Calhoun, V. D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 39 , 1666–1681 (2008).
Whitfield-Gabrieli, S. et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl Acad. Sci. USA 106 , 1279–1284 (2009).
Palaniyappan, L., Simmonite, M., White, T. P., Liddle, E. B. & Liddle, P. F. Neural primacy of the salience processing system in schizophrenia. Neuron 79 , 814–828 (2013).
Mittner, M., Hawkins, G. E., Boekel, W. & Forstmann, B. U. A neural model of mind wandering. Trends Cogn. Sci. 20 , 570–578 (2016). This paper convincingly argues for the introduction of two important novel elements to the scientific study of mind-wandering: employing cognitive modelling and a consideration of neuromodulatory influences on thought.
Fox, K. C. R. & Christoff, K. in The Cognitive Neuroscience of Metacognition (eds Fleming, S. M. & Frith, C. D.) 293–319 (Springer, 2014).
Foulkes, D. & Fleisher, S. Mental activity in relaxed wakefulness. J. Abnorm. Psychol. 84 , 66–75 (1975).
Fox, K. C. R., Nijeboer, S., Solomonova, E., Domhoff, G. W. & Christoff, K. Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Front. Hum. Neurosci. 7 , 412 (2013).
De Bono, E. Six Thinking Hats (Little Brown and Company, 1985).
Ellamil, M., Dobson, C., Beeman, M. & Christoff, K. Evaluative and generative modes of thought during the creative process. Neuroimage 59 , 1783–1794 (2012).
Beaty, R. E., Benedek, M., Kaufman, S. B. & Silvia, P. J. Default and executive network coupling supports creative idea production. Sci. Rep. 5 , 10964 (2015).
Fox, K. C. R., Kang, Y., Lifshitz, M. & Christoff, K. in Hypnosis and Meditation (eds Raz, A. & Lifshitz, M.) 191–210 (Oxford Univ. Press, 2016).
Fazelpour, S. & Thompson, E. The Kantian brain: brain dynamics from a neurophenomenological perspective. Curr. Opin. Neurobiol. 31 , 223–229 (2015).
Download references
Acknowledgements
The authors are grateful to R. Buckner, P. Carruthers, M. Cuddy-Keane, M. Dixon, S. Fazelpour, D. Stan, E. Thompson, R. Todd and the anonymous reviewers for their thoughtful feedback on earlier versions of this paper, and to A. Herrera-Bennett for help with the figure preparation. K.C. was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) (RGPIN 327317–11) and the Canadian Institutes of Health Research (CIHR) (MOP-115197). Z.C.I. was supported by a Social Sciences and Humanities Research Council of Canada (SSHRC) postdoctoral fellowship, the Balzan Styles of Reasoning Project and a Templeton Integrated Philosophy and Self Control grant. K.C.R.F. was supported by a Vanier Canada Graduate Scholarship. R.N.S. was supported by an Alzheimer's Association grant (NIRG-14-320049). J.R.A.-H. was supported by a Templeton Science of Prospection grant.
Author information
Authors and affiliations.
Department of Psychology, University of British Columbia, 2136 West Mall, Vancouver, V6T 1Z4, British Columbia, Canada
Kalina Christoff & Kieran C. R. Fox
Centre for Brain Health, University of British Columbia, 2211 Wesbrook Mall, Vancouver, V6T 2B5, British Columbia, Canada
Kalina Christoff
Departments of Philosophy and Psychology, University of California, Berkeley, 94720, California, USA
Zachary C. Irving
Department of Human Development, Laboratory of Brain and Cognition, Cornell University,
R. Nathan Spreng
Human Neuroscience Institute, Cornell University, Ithaca, 14853, New York, USA
Institute of Cognitive Science, University of Colorado Boulder, UCB 594, Boulder, 80309–0594, Colorado, USA
Jessica R. Andrews-Hanna
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kalina Christoff .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5.
A mental state, or a sequence of mental states, including the transitions that lead to each state.
A transient cognitive or emotional state of the organism that can be described in terms of its contents (what the state is 'about') and the relation that the subject bears to the contents (for example, perceiving, believing, fearing, imagining or remembering).
Thoughts with contents that are unrelated to what the person having those thoughts is currently doing.
Thinking that is characteristically fanciful (that is, divorced from physical or social reality); it can either be spontaneous, as in fanciful mind-wandering, or constrained, as during deliberately fantasizing about a topic.
A thought with contents that are unrelated to the current external perceptual environment.
A deliberate guidance of current thoughts, perceptions or actions, which is imposed in a goal-directed manner by currently active top-down executive processes.
The emotional significance of percepts, thoughts or other elements of mental experience, which can draw and sustain attention through mechanisms outside of cognitive control.
Features of current perceptual experience, such as high perceptual contrast, which can draw and sustain attention through mechanisms outside of cognitive control.
The process of spontaneously or deliberately inferring one's own or other agents' mental states.
Flexible combinations of distinct elements of prior experiences, constructed in the process of imagining a novel (often future-oriented) event.
A type of dreaming during which the dreamer is aware that he or she is currently dreaming and, in some cases, can have deliberate control over dream content and progression.
The ability to produce ideas that are both novel (that is, original and unique) and useful (that is, appropriate and meaningful).
A method in which participants are probed at random intervals and asked to report on aspects of their subjective experience immediately before the probe.
Different ways of categorizing a thought based on its contents, including stimulus dependence (whether the thought is about stimuli that one is currently perceiving), task relatedness (whether the thought is about the current task), modality (visual, auditory, and so on), valence (whether the thought is negative, neutral or positive) or temporal orientation (whether the thought is about the past, present or future).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Christoff, K., Irving, Z., Fox, K. et al. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci 17 , 718–731 (2016). https://doi.org/10.1038/nrn.2016.113
Download citation
Published : 22 September 2016
Issue Date : November 2016
DOI : https://doi.org/10.1038/nrn.2016.113
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Evidence synthesis indicates contentless experiences in meditation are neither truly contentless nor identical.
- Toby J. Woods
- Jennifer M. Windt
- Olivia Carter
Phenomenology and the Cognitive Sciences (2024)
The mediating role of default mode network during meaning-making aroused by mental simulation between stressful events and stress-related growth: a task fMRI study
Behavioral and Brain Functions (2023)
The role of memory in creative ideation
- Mathias Benedek
- Roger E. Beaty
- Yoed N. Kenett
Nature Reviews Psychology (2023)
A tripartite view of the posterior cingulate cortex
- Brett L. Foster
- Seth R. Koslov
- Sarah R. Heilbronner
Nature Reviews Neuroscience (2023)
Effect of subconscious changes in bodily response on thought shifting in people with accurate interoception
- Mai Sakuragi
- Kazushi Shinagawa
- Satoshi Umeda
Scientific Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, towards a neuroscience of mind-wandering.
- 1 Functional Brain Center, Wohl Institute for Advanced Imaging, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel
- 2 Department of Psychology, Tel-Aviv University, Tel-Aviv, Israel
- 3 Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel
- 4 The Emotion-Cognition Research Center, Shalvata Mental Health Center, Hod-Hasharon, Israel
- 5 Department of Neurobiology, Weizmann Institute of Science, Rehovot, Israel
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. Nevertheless, the scientific exploration of MW poses unique challenges; MW is by nature a spontaneous, off task, internal mental process which is often unaware and usually difficult to control, document or replicate. Consequently, there is a lack of accepted modus operandi for exploring MW in a laboratory setup, leading to a relatively small amount of studies regarding the neural basis of MW. In order to facilitate scientific examination of MW the current review categorizes recent literature into five suggested strategies. Each strategy represents a different methodology of MW research within functional neuroimaging paradigms. Particular attention is paid to resting-state brain activity and to the “default-mode” network. Since the default network is known to exert high activity levels during off-task conditions, it stands out as a compelling candidate for a neuro-biological account of mind-wandering, in itself a rest-based phenomenon. By summarizing the results within and across strategies we suggest further insights into the neural basis and adaptive value of MW, a truly intriguing and unique human experience.
“Thoughts meander like a restless wind inside a letter box they tumble blindly as they make their way across the universe” John Lennon
Introduction
Mind-wandering (MW) refers to ongoing mentation which occurs spontaneously, and largely autonomously, whenever an awake individual is not engaged in a cognitively demanding task. Alternative names to the term “MW” ( Smallwood and Schooler, 2006 ; Mason et al., 2007 ) in past and recent literature include “day dreaming” ( Giambra, 1979 ), “task-unrelated images and thought” ( Giambra and Grodsky, 1989 ), “stimulus independent thought” ( Teasdale et al., 1995 ), “task-unrelated thought” ( Smallwood et al., 2003 ), “incidental self-processing” ( Gilbert et al., 2005 ), “inner speech” ( Morin, 2009 ), and “spontaneous thought” ( Christoff et al., 2008 ).
Conceptualized as a core element of what William James defined as the “stream of consciousness” ( James, 1892 ), MW, in various names and forms, has gained considerable attention in ancient and modern philosophy and in theoretical psychology. The robust, autonomous, and continual nature of this psychological process has led writers to suggest that rather than being an undesired lapse of attention to the external world (William James remarked, when he was accused of being absent-minded, that he was really just present-minded to his own thoughts; Barzun, 1983 ), MW must have an important adaptive value for healthy cognition ( Christoff et al., 2008 ; Baars, 2010 ). Yet much like the neural basis of MW, its adaptivity and the nature of its interaction with other cognitive processes remain a scientific blind spot.
In the relatively short history of cognitive neuroscience, which has inherited much of its models, paradigms, and findings from behavioral and cognitive psychology, MW is virtually absent ( Smallwood and Schooler, 2006 ) as a subject of research. The reluctance in the scientific arena to study MW can be accounted for by its non-behavioral characteristics when compared to more conventionally studied mental functions: MW occurs in the absence of any external cue; it is often unintended and even unaware; it takes its own course – probably driven by internally generated cues; and it is hard to trace back, replicate or report. However, a recent paradigm shift in functional neuroimaging holds a great promise for the development and establishment of MW research. The discovery of the “default-mode network” (DMN; Raichle et al., 2001 ) and the following realization of the significance of spontaneous resting-state neural activity ( Raichle, 2009 ) dramatically launched a prosperous path in the scientific exploration of MW.
Default-mode network relates to a functionally meaningful neural network, which includes the medial prefrontal cortex (MPFC), the precuneus, the posterior cingulate cortex, and the inferior parietal and lateral temporal cortices (Figure 1 ). In comparison to other functional neural networks, DMN has unique patterns of activity ( Gusnard et al., 2001 ; Raichle et al., 2001 ): both in terms of energy consumption and in terms of the blood oxygen-level dependent (BOLD) signal, activation levels in this network were shown to descend below baseline during cognitively demanding tasks. Moreover, this network shows high activation levels at rest compared to task. These activation patterns and their possible functional meaning have received considerable attention in recent years, using independent as well as combined neuroimaging techniques (e.g., Ben-Simon et al., 2008 ). Studies with clinical populations shed additional light on the critical functionality of the DMN by demonstrating that malfunctioning of the DMN is associated with several neurological, psychiatric, and psychological pathologies (for a review see Buckner et al., 2008 ).
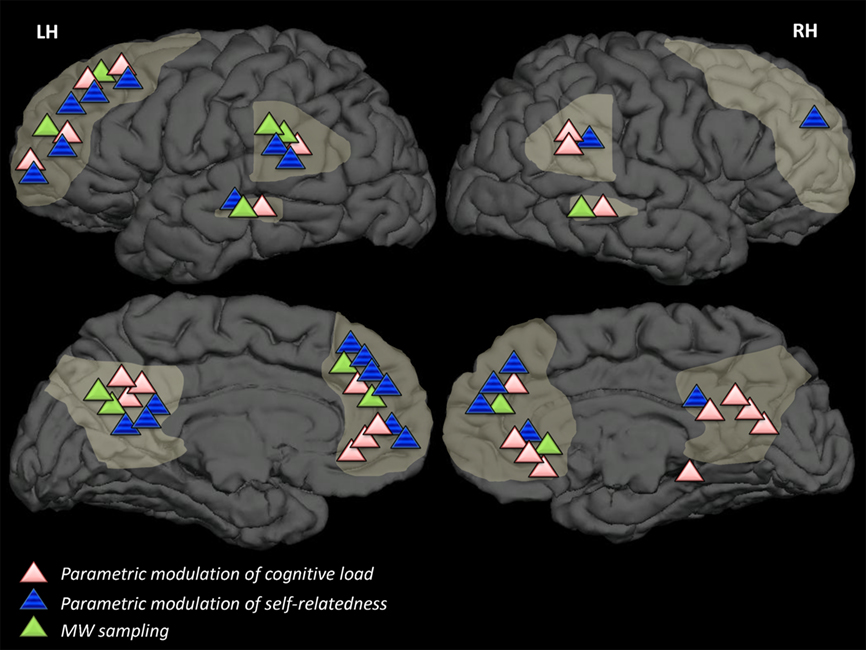
Figure 1. Results of overviewed studies in relation to DMN regions . DMN-related results of studies overviewed in this review, categorized by strategy, superimposed on a template brain. Light-grey markings denote DMN areas (in accordance with Buckner et al., 2008 ) dorsal and ventral medial prefrontal cortex, lateral temporal cortices, precuneus, and posterior cingulate cortex. Strategies which have not been employed in neuroimaging studies (strategy A2) or strategies which are concerned with degree of connectivity rather than degree of activation in brain areas (strategy B3) are not represented in this figure.
Since the first reports describing it, DMN rest-related activity had been suggested to comprise a neural correlate of MW ( Gusnard et al., 2001 ). This proposition was based on two main features of the DMN: First of all, like MW, DMN activity occurs during rest and shows a reverse correlation with cognitive load ( Mason et al., 2007 ). Secondly, task-related activations in the medial prefrontal and parietal areas, which comprise substantial elements of the DMN, have been shown to occur during self-related tasks ( Northoff and Bermpohl, 2004 ; Spreng et al., 2009 ). This has led writers to suggest that rest-related activations in these areas might subserve MW, in itself a process of self-related mentation ( Baars, 2010 ).
With the exceedingly growing body of information on neural activity in the wakeful, resting state, the shortage in accepted modus operandi regarding the scientific examination of MW has become a bottle neck, restraining further examination of the functionality of the DMN on the one hand and of the neural basis of MW on the other. However, several pioneering attempts have been made to study the relation between DMN activity and MW, yielding striking results. Converging the solutions to the challenge of quantifying and scientifically studying MW presented in these studies portrays an array of potential strategies to address the question of a DMN–MW association.
The current review aims to facilitate the scientific exploration of the neural correlates of MW by overviewing existing literature and defining, respectively, five methodological strategies for studying MW within a functional neuroimaging paradigm. Two of these strategies include direct measurements of MW (strategies A1 and A2), whether in real time – during rest or task performance, or retrospectively. Three additional strategies (Strategies B1, B2, and B3) rely on theoretical assumptions regarding MW and self related or cognitive functioning, as well as on the known functionality of networks emerging from connectivity analysis performed on data acquired during the resting state. Through the prism of these five strategies, we review existing literature and findings regarding MW published mainly in the recent decade. Each strategy will be presented in light of its advantages and disadvantages as well as the degree of its fitting to various paradigms and data analysis techniques in experimental neuroimaging.
Strategies for Studying the Neural Correlates of Mind-Wandering
The current section overviews methodologies and results from a representative sample of a decade of literature, mainly functional neuroimaging (PET or fMRI) studies, regarding the relation between DMN activation patterns and MW. The inclusion criterion for studies in this overview was that they bring forward the question of the relation between rest-related DMN activity and rest-related phenomenological experience. Importantly, studies of self-related functions were only included if they state a specific hypothesis regarding rest-related neural and psychological functioning. Table 1 lists the studies presented in this review, categorized by strategy. A visual illustration of the results obtained by these studies is presented in Figure 1 .
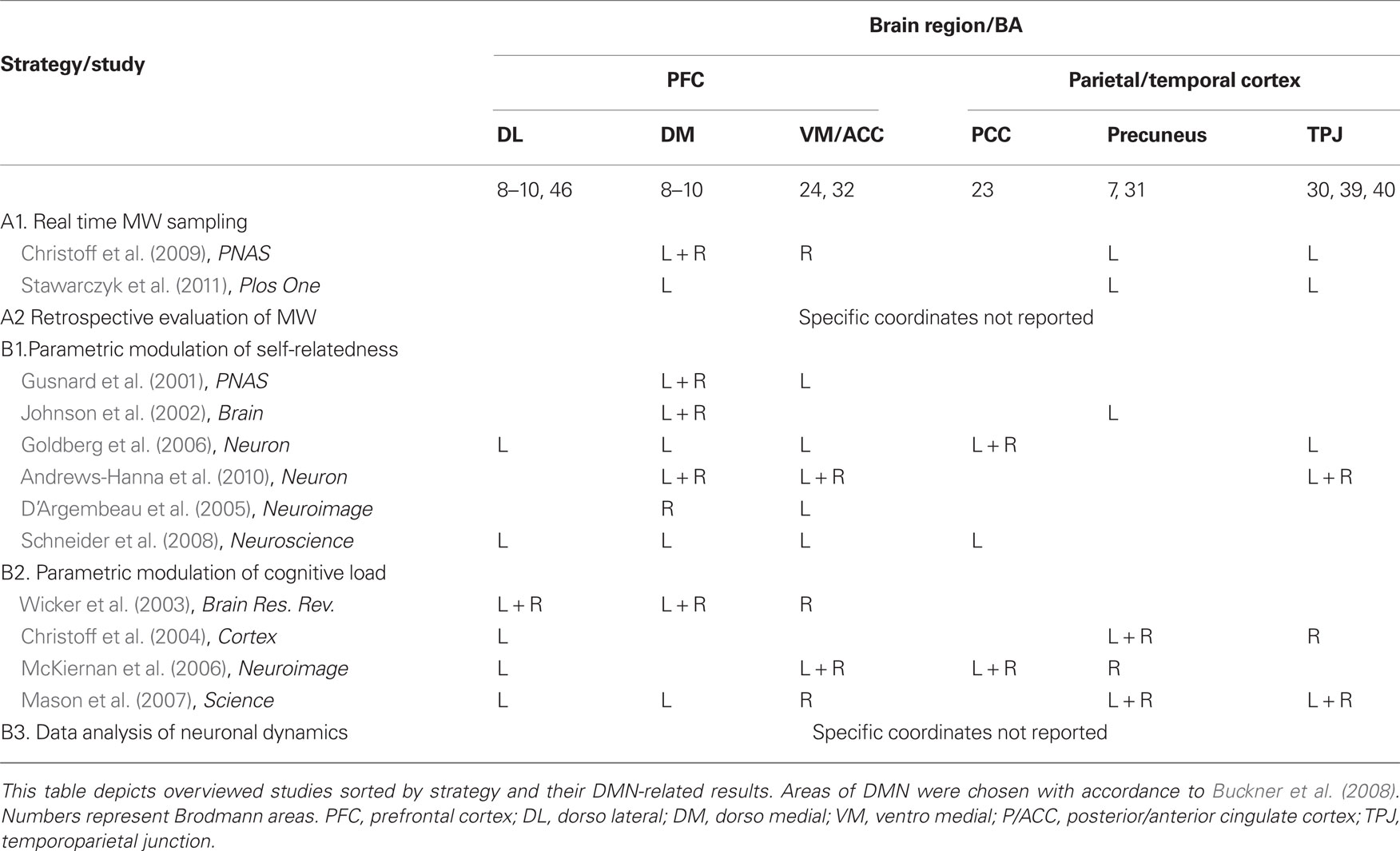
Table 1. A summary of overviewed studies and their DMN-related results.
The below differentiation between indirect and direct strategies could also be discussed in terms of determining the dependent and the independent variables within a functional neuroimaging setup: in the case of the indirect strategies, cognitive load or self-relatedness are being experimentally manipulated (i.e., independent variable) and are expected to cause a change in the measured neural signal of the DMN (i.e., dependent variable); in the case of the direct strategies, the degree of DMN activation is manipulated (i.e., independent variable) by altering rest and task while scanning, and the consequent change in the degree of MW is being assessed following scanning (i.e., dependent variable). Table A2 in Appendix summarizes typical dependent and independent variables according to each strategy.
Direct Strategies for Measuring Mind-Wandering
Strategies for directly quantifying the degree of MW represent a straightforward attempt to overcome its non-explicit nature, and essentially make conventional experimental methods applicable for studying it. For example, one can use the obtained degree of reported MW to categorize sessions or subjects into groups before analyzing, or to correlate it with the degree of activation in selected brain regions of interest or in the whole brain. The greatest challenge, however, is that in contrast to most behavioral measurements, the actual tracking of MW, or even its mere verbalizing in real time, tampers with its very occurrence ( Filler and Giambra, 1973 ): an individual busy with reporting her own MW is less free to engage in spontaneous MW comparing to when left to rest quietly. This can probably account for the relatively few studies which have attempted to directly quantify MW in the history of cognitive neuroscience, and possibly, for the even fewer methods developed to do so. Several quantifications techniques have nonetheless, been employed, some attempting at real-time assessment of the degree of MW while others focusing on post hoc questioning of subjects.
Strategy A1: real-time MW sampling
Mind-wandering can occur with or without awareness of its occurrence (“meta-awareness”; Christoff et al., 2009 ). Nevertheless, one can normally report if a thought was occurring in their mind or not, if interrupted and asked to do so at a given time point. This is the rational underlying the MW sampling (also known as “thought sampling” or “thought probing”) technique ( McKiernan et al., 2006 ; Mason et al., 2007 ; Christoff et al., 2009 ; Stawarczyk et al., 2011 ). Several approaches have been introduced for MW sampling in the neuroimaging set up, but a typical one uses a probing tone in even or uneven intervals, during either a rest or a task scan; subjects are instructed to indicate whether they were experiencing a spontaneous thought (i.e., unrelated to task performance) at the time the tone was presented (or, in a similar version, since the previous probe; Giambra, 1995 ). In block-design neuroimaging studies, each scan session is then scored according to the rate of “yes” answers given in it out of the overall number of tones presented in the session. The degree of MW occurrence is found to correlate with degree of neural activity in the DMN, as illustrated in Figure 1 . In ERP studies, EEG recordings adjacent to the pressings are analyzed separately for “on-task” vs. “off-task” reports. Using this method, Kam et al. (2011) demonstrated that the P1 component to a visual or auditory probe was reduced during off task, implying a reduction in sensory level processing during MW.
An interesting body of research based on this strategy examines the link between MW (referred to as “task-unrelated thought”) and errors during task performance ( Smallwood et al., 2003 , 2008 ). These studies demonstrate that when MW intrudes during task performance, and attentional lapses occur, task performance is impaired. Based on this line of research it may be suggested that MW competes with task performance on a limited capacity of attentional resources, in effect representing a state of “perceptual decoupling” ( Smallwood et al., 2011a ). This corresponds well with the idea discussed later on in this paper on the reverse correlation between MW and executive networks in the brain.
In yet another, less common, version of MW sampling, subjects are requested to press a button each time a thought comes into mind ( Giambra, 1989 ). Using this type of report, Braboszcz and Delorme (2010) asked subjects to press a button as soon as they realized their mind was wandering during a task of counting breaths. These presses were later used as an ERP analysis, showing reduced P200 responses to auditory stimuli, and reduced ability to identify the oddball auditory stimuli (smaller N100 during MW). In addition, frequency analysis showed that MW was associated with higher delta and theta power and lower alpha and beta power compared to task performance. Despite the suitability of this method for ERP studies, as reported by the authors themselves, this version seems to be less favorable and can hardly be found in neuroimaging studies, probably because it imposes greater meta-awareness and concentration from subjects and thus interferes with the natural occurrence of MW.
The strategy of MW sampling presents a clear advantage of being a real time, direct and quantified measurement of MW occurrence. One should bear in mind, though, that to the best of our knowledge it has never been systemically tested for validity and reliability, and thus it is mainly justified by its straight-forwardness and intuitiveness.
Strategy A2: retrospective evaluation of MW
Mind-wandering requires peace of mind; disturbances tend to interrupt its natural flow. In other words, an informative report regarding MW at a given time period, without interfering with its occurrence, may better be collected retrospectively, after a session has ended (notably, even then, the contents of MW is not always straightforwardly accessible to memory). Surprisingly, though, designated structured psychological questionnaires for explicitly assessing MW in healthy individuals are scarce. The very few examples which can be found in the literature ( Giambra, 1979 ; Klinger and Cox, 1987 ; Matthews et al., 1999 ) did not seem to survive the transition from psychological behavioral research to neuroscience. Consequently, and unfortunately, there is no accumulated body of literature regarding the neural basis of MW, and virtually no experience in the field obtained by retrospective questioning of MW using validated experimental instruments designated for this matter.
One inspiring study which could be considered an example for this approach is a PET study by D’Argembeau et al. (2005) . In this study subjects had to rate the total amount of thoughts experienced, whatever their content, using an in-house developed questionnaire immediately following scanning (a similar approach is found earlier in Mcguire et al. 1996 ). An alternative to using in-house developed questionnaires is to use established questionnaires of experiences which according to theoretical and clinical literature are related to MW. In such a study ( Gruberger et al., 2008 ), questionnaires for measuring dependent self awareness and degree of dissociation were applied to assess the degree of interference in MW during rest. The underlying hypothesis was that artificial interference with the normal process of MW will manifest itself as disruption in self awareness and as a sense of dissociation, which indeed was corroborated by the results. A third noteworthy example is the Resting State Questionnaire (ReSQ) published recently by Delamillieure et al. (2010) explicitly for usage in a functional neuroimaging setup. The ReSQ consists of 62 items organized by five main types of mental activity: visual mental imagery, inner language, somatosensory awareness, inner musical experience, and mental manipulation of numbers. Using a 0–100% scale, the participant retrospectively and quantitatively rates the proportion of time spent in each mental activity during the resting-state fMRI acquisition. Whether this tool will or will not eventually gain the confidence of the research community, its great importance lies in that it represents a pioneering effort to encompass the richness and individual nature of MW into a standardized questionnaire.
Indirect Strategies for Measuring Mind-Wandering
Indirect strategies – strategies in which MW is not directly measured – are typically based on the conceptualization of MW as self-related and as more prevalent during rest than during tasks of high cognitive demand. The hypothesis could be framed as follows: if DMN neural activity during rest is the neural basis of MW, then DMN activations during rest and during a given task should be more similar when the task shares common characteristics to MW, i.e., is characterized by low cognitive load and high self relevance.
The advantage of the indirect strategies is straightforward: they avoid measuring MW directly, thus overcoming its non-quantifiable nature and the lack of validated behavioral MW measures. Instead, they use accepted task-related behavioral measures (mostly validated or previously published) and modulate their self-relatedness or their degree of cognitive load.
Strategy B1: parametric modulation of self-relatedness
James’s “spiritual self” ( James, 1892 ), Gallagher’s “narrative self” ( Gallagher, 2000 ), Dennett’s “non-minimal self” ( Dennett, 1991 ), and Damasio’s “autobiographical self” ( Damasio, 1998 ), are just a few examples of how MW is often present within theoretical models of the self. It is typically represented as a module of its own, distinct both from “lower,” more basic, senses of consciousness as well as from “higher” self-related executive functions. Contemporary neuro-scientists also tend to agree that the “stream of consciousness” is inseparable from the ongoing, constant, sense of self ( Damasio, 1998 ; Gusnard, 2005 ; Beer, 2007 ). According to this notion, MW, whether its content is directly related to the thinker or not, is a self-related, self-generated, self-sustaining function ( Baars, 2010 ); it serves as an integral part of self awareness, a pre-requisite for healthy psychological functioning.
The conceptualization of MW as a private case of self-related functioning produces a hypothesis for an overlap between the neural basis of self-related tasks and the neural basis of MW. This hypothesis has been translated in some studies into a rational for comparing neural activations during self-related tasks to neural activations at rest, when MW is assumed to occur most.
Though not the first to suggest a relation between rest-related neural activity and MW, the first paper to specifically associate MW with DMN activity was published by Gusnard et al. (2001) , as part of a series of publications ( Raichle et al., 2001 ) introducing the concept of the DMN. In this fMRI study, neural activations during rest were compared both to a subjective, emotional judgment task (“internally cued condition”) and to a neutral judgment task (“externally cued condition”). In accordance with the above prediction, neural activations in DMN-related PFC areas were found to be more similar to the activations at rest during the internally cued condition than during the externally cued condition (see Table 1 for summarized results). Paradigms similar in contrasting a self-related task with a similar non-self-related task can be found in additional fMRI studies ( Johnson et al., 2002 ; Goldberg et al., 2006 ; Schneider et al., 2008 ; Andrews-Hanna et al., 2010 ), and in the PET study described earlier ( D’Argembeau et al., 2005 ). Results in all of these studies indicate greater activations (or lesser de-activations) in brain areas associated with the DMN, mostly MPFC areas, during self-related tasks than during non-self-related tasks, when compared to rest. These elevated activations were shown to last beyond the duration of the stimuli and into the rest period following stimulation ( Schneider et al., 2008 ). The majority of these papers (except for Johnson et al., 2002 ) demonstrate that DMN activations during self-related conditions were more similar to DMN rest-related activity patterns, and suggest that this result might imply a possible functional role of rest-related DMN activations in spontaneous self-related mental activity.
As shown in separate studies as well as in convergence, this is a useful strategy for investigating the functional role of areas within the DMN while staying within the boundaries of accepted neuroimaging paradigms. One drawback of this strategy is the potential of over stretching the concept of self, which may cause confounding the self-relatedness of a task with other characteristics like its emotional valence (e.g., Gusnard et al., 2001 ). Therefore, one should pay special attention that the parameter modulated between study conditions is indeed as specific to self-relatedness as possible.
Strategy B2: parametric modulation of cognitive load
The distinction of ongoing spontaneous mentation from other, task related, mental functions dates back to James (1892) , and has been recognized almost solely by theoretical psychology and philosophy over the years ( Gallagher, 2000 ). However, this very classification of MW as the mental function characterizing the un-engagement of attentional resources directly magnifies its potential to be scientifically explored.
The strategy of parametric modulation of cognitive load has been used in the context of studying the functionality of rest-related DMN activity. In this strategy, the contrast of interest when analyzing imaging data is not the commonly used task minus rest contrast, but rather the contrast of rest minus task. Researchers try to demonstrate that the lower the cognitive load in a given task condition, the higher the activations in DMN areas during this task, leading to a smaller difference between DMN activations during the task compared to rest. Indeed, this was found to be the case in fMRI studies such as McKiernan et al. (2006) , Christoff et al. (2004) , and Mason et al. (2007) , and in Wicker et al.’s (2003) meta-analysis of PET studies. In the case of McKiernan et al. (2006) and Mason et al. (2007) , behavioral measures (described in strategy A1) were added to the study to further establish a more direct association between high DMN activations during low cognitive demand and MW.
This strategy yields results which correspond well with theoretical accounts of MW as well as with the lay intuition that MW is the “default” mental state when the mind is free to engage in it. In addition to its intuitiveness, and thus its simplicity, the advantage of this strategy is in its robustness: it was found to be replicated across virtually any behavioral task tested ( Shulman et al., 1997 ; Mazoyer et al., 2001 ; Wicker et al., 2003 ), which makes it accessible and easy to implement. It should be taken into account, however, that executive functioning and MW are probably not as anti-correlated as these studies may depict. MW may involve executive processes like memory, planning, computing, etc., as is reflected by findings of executive networks co-activated with DMN during MW ( Christoff et al., 2009 ). Thus, rather than assuming mutual exclusiveness, the degree and direction of the association between neural activity of the DMN and of executive networks during MW should be studied in greater experimental resolution.
Strategy B3: paradigm-free analysis of neuronal dynamics
Brain activity is combined of activations of neurons which comprise anatomical and functional networks. Recent advances in functional and computational neuroimaging have provided new tools for examining functional interactions between time series of signals obtained from different brain regions, catalyzing the examination of functional connectivity in the resting brain. This type of analysis does not require a behavioral paradigm (“paradigm-free”) and indeed is often implemented on data acquired solely when subjects lie resting in the imaging device (the validity of these signals is discussed in Box 1). In fMRI, analysis methods of the resting-state signal can typically be placed into hypothesis dependent and hypothesis free methods ( Van Den Heuvel and Hulshoff Pol, 2010 ), both resulting in connectivity maps – whether correlational or anti-correlational ( Uddin et al., 2009 ). These maps demonstrate anatomical networks which, interestingly, greatly overlap with known functional neural networks. The DMN is one of those emerging networks and thus its relation to MW can be further characterized in terms of functional connectivity.
Box 1. Validation of spontaneous BOLD fluctuations acquired during rest.
The neuronal basis of spontaneous resting-state fMRI signals was initially regarded by skeptics as problematic, potentially representing merely unknown parameters of noise as well as known physiological ones. However recent observations increasingly support and validate the neuronal basis of resting-state fMRI signals (Adapted from Van Den Heuvel and Hulshoff Pol, 2010 ):
• The first and probably most compelling evidence for the resting-state signal is that most resting-state patterns tend to occur between brain regions overlapping in known functional and neuroanatomical regions ( Salvador et al., 2005 ; Damoiseaux et al., 2006 ; Van Den Heuvel et al., 2008 ).
• The second observation relates to the frequency of rest-related signals revealing that the observed spontaneous BOLD signals are mainly dominated by lower frequencies (<0.1 Hz) with only a minimal contribution of higher frequency cardiac and respiratory oscillations (>0.3 Hz) ( Cordes et al., 2000 , 2001 ).
• Lastly, an (indirect) association exists between the frequency profiles of slow spontaneous resting-state fMRI and electrophysiological recordings of neuronal firing ( Nir et al., 2008 ) and between spontaneous BOLD fluctuations and simultaneous measured fluctuations in neuronal spiking ( Shmuel et al., 2002 ; Shmuel and Leopold, 2008 ).
Altogether these findings advocate toward the validity of the neural signal acquired during the resting state and the legitimacy of its scientific exploration.
Two studies are brought here to exemplify the usage of a paradigm-free strategy in further characterizing the relation between MW and DMN spatio-temporal dynamics. Horovitz et al. (2008) utilized this strategy to determine whether DMN activity can be de-coupled from conscious awareness. In this study, the level of functional connectivity within the DMN persisted both during the resting state and during light sleep. The authors conclude that DMN connectivity “does not require or reflect the level of consciousness that is typical for wakefulness” (p. 679), which seems to undermine the idea of a functional involvement of DMN activity in MW. Nevertheless, two alternative explanations are offered by the authors: the first is that these results only decouple wakeful awareness from the degree of connectivity within the DMN, but not from the amplitude of its activity ; the other is that light sleep is sometimes characterized by the existence of dream-like reverie activity (a mental activity similar to MW) which like MW may also be a functional product of DMN activity.
Another study by the same group ( Horovitz et al., 2009 ) demonstrated altered correlations between DMN network components during different states of consciousness, most notably a reduced involvement of the MPFC during sleep. The authors suggest that among the DMN components, the frontal cortex may play an important role in the sustenance of conscious awareness.
In favor of this strategy, it can be claimed that as some indication exists for the effect of previous task performance on neural activity at subsequent rest ( Northoff et al., 2010 ), a paradigm-free study design which consists of rest alone will produce results which are more unbiased. In any case, studies of this strategy call attention to the fact that beyond relative degree of neural activity, more holistic parameters of neural dynamics need to be explored to truly characterize the DMN–MW relation, such as temporal and spatial patterns of DMN activity.
Discussion and Future Directions
In this review we portray the evolvement of the neuroscience of MW, in hope to lay the grounds for additional research to come. Undoubtfully, studies like the ones overviewed here serve to narrow the gap between theoretical understanding of MW and its scientific exploration. Nevertheless, MW is still by large a mystery, and much work remains to complete the puzzle. In Box 2 we put forward several ideas which stem from existing findings in hope of contributing to future research.
Box 2. Mind-wandering: questions for future research.
Understanding MW using brain imaging techniques holds a promise for this field of research. Listed here are a few lines of thought that could constitute an initial framework for future MW studies:
A. Temporal patterns of MW: What are the spatio-temporal dynamics which correspond to MW in the human brain? How are they represented in terms of brain connectivity?
B. Control of MW: what is MW’s locus of control in the brain? Do internal and external abruptions of MW result in similar neural outcome? Interfering with MW occurrence by different type of tasks (e.g., tasks which require external vs. internal attention) could offer preliminary answers.
C. MW and consciousness: What is the nature of the relationship between consciousness and MW? Is MW simply an expression of conscious experience much like an actor on a stage or is it a substantial part of consciousness giving rise to the stage itself? If MW is indeed a substantial part of conscious experience one would expect similar neural correlates of both phenomena.
D. MW and pathologies: Which functions does MW serve and how are they disrupted when MW does not occur? Both the very mechanism and the contents of MW are of great interest to clinical psychology and psychiatry. Psychiatric and neuronal pathologies associated with MW malfunctioning may shed light on understanding the role of MW in healthy psychological functioning.
E. The contents of MW: In this review we put little emphasis on the ever changing contents of MW. This is not to say that they are of no importance, only that the studies described here were interested in the common mechanism underlying this changing flow of contents. Future research might very well attempt to segregate neural patterns during MW which are responsible for the experience of different contents or even different time directions (e.g., future or past) as explored by Smallwood et al. (2009) .
To begin answering such questions, the scientific community must agree upon theoretical definitions as well as normalized, standardized behavioral measures of MW. In the functional neuroimaging field one also needs advanced validated computational methods for studying the temporal dynamics of neural activations in long sequences such as common in rest.
Mind-wandering can be studied under different contexts involving a wide array of experimental questions. Accordingly, as we tried to exemplify in this review, there is no absolute optimal way to study it, but rather it is important to make an informed, educated choice when studying it within a neuroimaging paradigm. For instance, MW sampling provides valuable information about inter-subject and intra-subject differences in the degree of MW, while sacrificing the integrity of its natural, untouched flow; In contrast parametric modulation of cognitive load does not interfere with the natural course of MW and also enables statistical analysis of inter-group variance, with the compromise of MW being only implied, and not directly measured. Figure 2 depicts a flow chart of relevant considerations in making the most advantageous choice for a given experimental setup.
Figure 2. Flowchart of goodness of fit of different strategies according to study aims . A flowchart which may assist researchers wishing to explore mind-wandering using functional neuroimaging paradigms. According to study aims one should decide on the appropriate strategy taking into consideration advantages and disadvantages of each strategy as discussed in the text.
In addition to portraying modes of operation for the scientific examination of MW, overviewing the neuroscience of MW so far provides a few insights into this neural and mental process.
Functionality of Mind-Wandering
The robustness of the experience of MW across ages, cultures, and individuals ( Singer and McCraven, 1961 ), suggests it holds a vital role in human psychology. As to the specific role of MW, we suggest several ideas based on current literature which may inspire future research.
MW serves “self” functions
As detailed in the context of strategy B1, there are theoretical ( Gallagher, 2000 ), neuroanatomical ( Gusnard, 2005 ; Northoff et al., 2006 ), and intuitive grounds to claim that MW is a self-related cognitive function, which serves to create and maintain an integrated, meaningful sense of self out of various aspects of self-related information and cognition. Northoff et al. (2006) , for instance, conceptualizes MW as a “psychological baseline,” a form of continuous self-referential processing which is evident during non-task conditions and which ultimately forms our subjective experience of a “continuous stream of subjective experience” or “phenomenal time” where past, present, and future are no longer divided but integrated.
MW enables the projection of a “self” to past and future events
The idea that MW serves processes of future planning and simulation is based on theory and common experience, and is strongly supported by the fact that the DMN includes areas such as the posterior cingulate cortex, the precuneus, and the hippocampus, which are known to take part in such mental processes ( Buckner et al., 2008 ). Behaviorally, it has been shown that the contents of MW will tend toward prospecting or retrospecting according to the self relevance of a given context ( Smallwood et al., 2009 ), suggesting that MW serves to integrate past and present experiences for the purpose of future planning. Moreover, Smallwood et al. (2011b) suggest that self reflection associated with future-oriented thinking is an integral part of the autobiographical memory system. Interestingly, temporal locus of MW has even been shown to be related to the direction of apparent physical movement through space (forward/backward), implying a functional link between MW temporality and sensory spatio-temporal input ( Miles et al., 2010 ).
Altogether, this idea corresponds well with Tulving’s idea of “autonoetic consciousness,” which is claimed to be selective to the human kind and which enables mentally traveling into the past and the future ( Tulving, 2005 ).
MW serves as a learning and consolidation mechanism by augmenting the associative abilities of the brain
According to this proposition, spontaneous mental processing during wakefulness resembles in its function, in its effects and, to a certain extent, in its neural basis, the off-line processing that occurs during sleep. This relatively recent idea is presented by contemporary writers ( Christoff et al., 2008 ; Baars, 2010 ) and already takes into account what is known about DMN activation patterns. According to this notion, it could be suggested that task performance would improve following MW in a similar way when following sleep ( Stickgold et al., 2001 ).
Mind-Wandering-Executive Functioning Relation: An Integrative Approach
Converging results from studies like the ones overviewed here provide verification for a strong negative association between MW and executive functioning. This association, mentioned earlier to be part of the rational for strategy B2 (Parametric modulation of cognitive load), is supported by behavioral as well as neuro-scientific evidence (e.g., DMN activity). In light of the infancy of MW research, this in itself is a highly instrumental insight.
Nevertheless, recent lines of evidence suggest that this association is not exclusive. The first is found in the activation of executive prefrontal and parietal brain areas, in addition to DMN areas, as contributing to MW ( Christoff et al., 2009 ). The second is found in the gradual increase of DMN activity found in strategies B1 and B2 as cognitive load decreases and self-relatedness increases, which suggests that some DMN activity did occur even in lower self relevance or higher cognitive load conditions. The third line of evidence is brought by studies which show involvement of DMN areas during online task performance ( Assaf et al., 2009 ).
Though assuming a dichotomy between MW and “executive” neural networks proved useful for the beginning of MW research, a more mature approach might suggest studying the interplay between MW and executive functions and their underlying neural mechanisms ( Smallwood and Schooler, 2006 ). In consistence with this line of thought, Spreng et al. (2010) suggest that a third anatomically interposed “frontoparietal control network” mediates planning across domains, flexibly coupling with either the default or dorsal attention network in support of internally vs. externally focused cognition, respectively.
Rather than eliminating them, MW probably serves various cognitive functions such as prospective planning, self monitoring, etc. ( Baars, 2010 ). A better understanding of the interplay between MW and executive functioning can be achieved by further implementation of the five strategies defined here, in turn contributing altogether to the understanding of the adaptive value of MW with respect to human cognition and affect.
Mind-Wandering: The Neural Basis of Its Integration and Segregation
Portraying the results of the overviewed studies suggests that MW involves activities in distributed brain areas (see Table 1 and Table A1 in Appendix). These findings of different activations might underlie specific aspects of the MW process and in turn may serve to deconstruct MW, both theoretically and operationally, into elements according to its content or to the additional mental functions which are involved in it (e.g., emotion, autobiographical memory, mental time traveling, etc.). Examining the different DMN activations according to strategy, as illustrated in Figure 1 , implies that some sub-areas within the DMN are common to MW in any context while others are more typically unique to a specific strategy. For example, on an impressionist level only, it could be suggested that across strategies lateral correlates of MW are found more dominantly in the left than in the right hemisphere and can be commonly regarded as part of the network associated with high-level semantic processing. However other correlates of MW do differ between strategies, with the ventral MPFC and precuneus more sensitive to modulation of cognitive load, and dorso-medial MPFC areas more sensitive to self-relatedness.
It is of no doubt that such impressions require a comprehensive quantitative meta-analysis which is beyond the scope of this review. Nevertheless, such a neuro-functional differentiation implies that each strategy might reveal, in addition to the network underlying MW, the neural basis of a specific aspect within the large construct of MW. A functionally based deconstruction of the DMN has already been suggested ( Spreng et al., 2009 ; Andrews-Hanna et al., 2010 ; Stawarczyk et al., 2011 ) and could prove fruitful for further scientific examination of MW; Similar MW studies utilizing such refined and specific definitions may shed additional light on differential neural processes which underlie diverse aspects of MW.
Concluding Remarks
Mind-wandering is a universal phenomenon which accompanies much of our daily lives from childhood to adulthood. Its exploration has a vast potential in leading us to a better and more profound understanding of our ongoing mental selves, and in fact, of the basic properties of conscious experience.
The study of MW is at an exciting position of forming into a field of research of its own. Its relevance to a wide array of disciplines, from neuroscience to philosophy to the clinical world ensures that it will draw a growing number of researchers in the near future. We hope that this review serves to set the milestones for a better scientific understanding of this remarkable, unique human quality.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., and Buckner, R. L. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550.
Pubmed Abstract | Pubmed Full Text
Assaf, M., Kahn, I., Pearlson, G. D., Johnson, M. R., Yeshurun, Y., Calhoun, V. D., and Hendler, T. (2009). Brain activity dissociates mentalization from motivation during an interpersonal competitive game. Brain Imaging Behav. 3, 24–37.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Baars, B. J. (2010). Spontaneous repetitive thoughts can be adaptive: postscript on “mind wandering”. Psychol. Bull. 136, 208–210.
Barzun, J. (1983). A Stroll with William James. New York, NY: HarperCollins.
Beer, J. S. (2007). The default self: feeling good or being right? Trends Cogn. Sci. (Regul. Ed.) 11, 187–189.
Ben-Simon, E., Podlipsky, I., Arieli, A., Zhdanov, A., and Hendler, T. (2008). Never resting brain: simultaneous representation of two alpha related processes in humans. PLoS ONE 3, e3984. doi: 10.1371/journal.pone.0003984
Braboszcz, C., and Delorme, A. (2010). Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage 54, 3040–3047.
Buckner, R., Andrews-Hanna, J., and Schacter, D. (2008). The brain’s default network. Ann. N. Y. Acad. Sci. 1124, 1–38.
Christoff, K., Gordon, A., Smith, R., and Vancouver, B. C. (2008). “The role of spontaneous thought in human cognition,” in Neuroscience of Decision Making , ed. O. A. M. Vartanian (Philadelphia: Psychology Press).
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., and Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U.S.A. 106, 8719–8724.
Christoff, K., Ream, J. M., and Gabrieli, J. D. (2004). Neural basis of spontaneous thought processes. Cortex 40, 623–630.
Cordes, D., Haughton, V. M., Arfanakis, K., Carew, J. D., Turski, P. A., Moritz, C. H., Quigley, M. A., and Meyerand, M. E. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 22, 1326.
Cordes, D., Haughton, V. M., Arfanakis, K., Wendt, G. J., Turski, P. A., Moritz, C. H., Quigley, M. A., and Meyerand, M. E. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 21, 1636.
D’Argembeau, A., Collette, F., Van Der Linden, M., Laureys, S., Del Fiore, G., Degueldre, C., Luxen, A., and Salmon, E. (2005). Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage 25, 616–624.
Damasio, A. R. (1998). Investigating the biology of consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1879–1882.
Damoiseaux, J. S., Rombouts, S., Barkhof, F., Scheltens, P., Stam, C. J., Smith, S. M., and Beckmann, C. F. (2006). Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U.S.A. 103, 13848.
Delamillieure, P., Doucet, G., Mazoyer, B., Turbelin, M.-R., Delcroix, N., Mellet, E., Zago, L., Crivello, F., Petit, L., Tzourio-Mazoyer, N., and Joliot, M. (2010). The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res. Bull. 81, 565–573.
Dennett, D. C. (1991). Consciousness Explained. Boston: Little Brown & Co.
Filler, M. S., and Giambra, L. M. (1973). Daydreaming as a function of cueing and task difficulty. Percept. Mot. Skills 37, 503.
Gallagher, S. (2000). Philosophical conceptions of the self: implications for cognitive science. Trends Cogn. Sci. (Regul. Ed.) 4, 14–21.
Giambra, L. M. (1979). Sex differences in daydreaming and related mental activity from the late teens to the early nineties. Int. J. Aging Hum. Dev. 10, 1–34.
Giambra, L. M. (1989). Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol. Aging 4, 136–143.
Giambra, L. M. (1995). A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Conscious. Cogn. 4, 1–21.
Giambra, L. M., and Grodsky, A. (1989). Task-unrelated images and thoughts while reading. Imagery Curr. Perspect. 26–31.
Gilbert, S. J., Frith, C. D., and Burgess, P. W. (2005). Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur. J. Neurosci. 21, 1423–1431.
Goldberg, I. I., Harel, M., and Malach, R. (2006). When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 50, 329–339.
Gruberger, M., Hendelr, T., Harel, E. V., Harari, H., Levkovitz, Y., and Zangen, A. (2008). I think therefore I am: alterations in the sense of self by stimulation of the prefrontal cortex. NeuroImage , 41, S41–S180.
Gusnard, D. A. (2005). Being a self: considerations from functional imaging. Conscious. Cogn. 14, 679–697.
Gusnard, D. A., Akbudak, E., Shulman, G. L., and Raichle, M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 4259–4264.
Horovitz, S. G., Braun, A. R., Carr, W. S., Picchioni, D., Balkin, T. J., Fukunaga, M., and Duyn, J. H. (2009). Decoupling of the brain’s default mode network during deep sleep. Proc. Natl. Acad. Sci. U.S.A. 106, 11376.
Horovitz, S. G., Fukunaga, M., De Zwart, J. A., Van Gelderen, P., Fulton, S. C., Balkin, T. J., and Duyn, J. H. (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG fMRI study. Hum. Brain Mapp. 29, 671–682.
James, W. (1892). Psychology. Cleveland: World.
Johnson, S. C., Baxter, L. C., Wilder, L. S., Pipe, J. G., Heiserman, J. E., and Prigatano, G. P. (2002). Neural correlates of self reflection. Brain 125, 1808–1814.
Kam, J. W. Y., Dao, E., Farley, J., Fitzpatrick, K., Smallwood, J., Schooler, J. W., and Handy, T. C. (2011). Slow fluctuations in attentional control of sensory cortex. J. Cogn. Neurosci. 23, 460–470.
Klinger, E., and Cox, W. M. (1987). Dimensions of thought flow in everyday life. Imagin. Cogn. Pers. 7, 125–128.
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., and Macrae, C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395.
Matthews, G., Joyner, L., Gilliland, K., Campbell, S. E., Falconer, S., and Huggins, J. (1999). Validation of a comprehensive stress state questionnaire: towards a state “big three”. Personal. Psychol. Eur. 7, 335–350.
Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L., and Tzourio-Mazoyer, N. (2001). Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res. Bull. 54, 287–298. doi: 10.1371/journal.pone.0018298
Mcguire, P. K., Paulsau, E., Frackowiak, R. S., and Frith, C. D. (1996). Brain activity during stimulus independent thought. Neuroreport 7, 2095–2099.
McKiernan, K. A., D’angelo, B. R., Kaufman, J. N., and Binder, J. R. (2006). Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage 29, 1185–1191.
Miles, L. K., Karpinska, K., Lumsden, J., and Macrae, C. N. (2010). The meandering mind: vection and mental time travel. PLoS ONE 5, e10825. doi: 10.1371/journal.pone.0010825
Morin, A. (2009). “Inner speech and consciousness,” in The Encyclopedia of Consciousness , ed. W. Banks (NY: Elsevier), 389–402.
Nir, Y., Mukamel, R., Dinstein, I., Privman, E., Harel, M., Fisch, L., Gelbard-Sagiv, H., Kipervasser, S., Andelman, F., and Neufeld, M. Y. (2008). Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat. Neurosci. 11, 1100–1108.
Northoff, G., and Bermpohl, F. (2004). Cortical midline structures and the self. Trends Cogn. Sci. (Regul. Ed.) 8, 102.
Northoff, G., Heinzel, A., De Greck, M., Bermpohl, F., Dobrowolny, H., and Panksepp, J. (2006). Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neuroimage 31, 440–457.
Northoff, G., Qin, P., and Nakao, T. (2010). Rest-stimulus interaction in the brain: a review. Trends Neurosci. 33, 277–284.
Raichle, M. E. (2009). A paradigm shift in functional brain imaging. J. Neurosci. 29, 12729–12734.
Raichle, M. E., Macleod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682.
Salvador, R., Suckling, J., Schwarzbauer, C., and Bullmore, E. (2005). Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 937.
Schneider, F., Bermpohl, F., Heinzel, A., Rotte, M., Walter, M., Tempelmann, C., Wiebking, C., Dobrowolny, H., Heinze, H. J., and Northoff, G. (2008). The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 157, 120–131.
Shmuel, A., and Leopold, D. A. (2008). Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum. Brain Mapp. 29, 751–761.
Shmuel, A., Yacoub, E., Pfeuffer, J., Van De Moortele, P. F., Adriany, G., Hu, X., and Ugurbil, K. (2002). Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36, 1195–1210.
Shulman, G. L., Corbetta, M., Buckner, R. L., Fiez, J. A., Miezin, F. M., Raichle, M. E., and Petersen, S. E. (1997). Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J. Cogn. Neurosci. 9, 624–647.
Singer, J. L., and McCraven, V. G. (1961). Some characteristics of adult daydreaming. J. Psychol. 51, 151–164.
CrossRef Full Text
Smallwood, J., Brown, K. S., Tipper, C., Giesbrecht, B., Franklin, M. S., Mrazek, M. D., Carlson, J. M., and Schooler, J. W. (2011a). Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS ONE 6, e18298.
Smallwood, J., Schooler, J. W., Turk, D. J., Cunningham, S. J., Burns, P., and Macrae, C. N. (2011b). Self-reflection and the temporal focus of the wandering mind. Conscious. Cogn. (in press).
Smallwood, J., Mcspadden, M., and Schooler, J. W. (2008). When attention matters: the curious incident of the wandering mind. Mem. Cognit. 36, 1144.
Smallwood, J., Nind, L., and O’connor, R. C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Conscious. Cogn. 18, 118–125.
Smallwood, J., and Schooler, J. W. (2006). The restless mind. Psychol. Bull. 132, 946.
Smallwood, J. M., Baracaia, S. F., Lowe, M., and Obonsawin, M. (2003). Task unrelated thought whilst encoding information. Conscious. Cogn. 12, 452–484.
Spreng, R. N., Mar, R. A., and Kim, A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510.
Spreng, R. N., Stevens, W. D., Chamberlain, J. P., Gilmore, A. W., and Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317.
Stawarczyk, D., Majerus, S., Maquet, P., and D’Argembeau, A. (2011). Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS ONE 6, e16997. doi: 10.1371/journal.pone.0016997
Stickgold, R., Hobson, J. A., Fosse, R., and Fosse, M. (2001). Sleep, learning, and dreams: off-line memory reprocessing. Science 294, 1052.
Teasdale, J. D., Dritschel, B. H., Taylor, M. J., Proctor, L., Lloyd, C. A., Nimmo-Smith, I., and Baddeley, A. D. (1995). Stimulus-independent thought depends on central executive resources. Mem. Cognit. 23, 551–551.
Tulving, E. (2005). Episodic Memory and Autonoesis: Uniquely Human? New York: Oxford University Press.
Uddin, L. Q., Clare Kelly, A., Biswal, B. B., Xavier Castellanos, F., and Milham, M. P. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 30, 625.
Van Den Heuvel, M., Mandl, R., and Pol, H. H. (2008). Normalized cut group clustering of resting-state FMRI data. PLoS ONE 3, e2001. doi: 10.1371/journal.pone.0002001
Van Den Heuvel, M. P., and Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534.
Wicker, B., Ruby, P., Royet, J.-P., and Fonlupt, P. (2003). A relation between rest and the self in the brain? Brain Res. Rev. 43, 224.
Keywords: mind-wandering, default-mode network, self, resting state, neuroimaging, fMRI, task independent thought, stimulus independent thought
Citation: Gruberger M, Ben-Simon E, Levkovitz Y, Zangen A and Hendler T (2011) Towards a neuroscience of mind-wandering. Front. Hum. Neurosci. 5 :56. doi: 10.3389/fnhum.2011.00056
Received: 08 January 2011; Accepted: 25 May 2011; Published online: 06 June 2011.
Reviewed by:
Copyright: © 2011 Gruberger, Ben-Simon, Levkovitz, Zangen and Hendler. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Talma Hendler, Functional Brain Center, Wohl Institute for Advanced Imaging, Tel-Aviv Sourasky Medical Center, 6 Weizmann Street, Tel-Aviv 64239, Israel. e-mail: talma@tasmc.health.gov.il

META Lab | Psychological & Brain Sciences | UC Santa Barbara
Mind wandering.
Our mind-wandering research can be roughly divided into five categories.
Related Questions
Selected publications.
- Unaware yet reliant on attention: Experience sampling reveals that mind-wandering impedes implicit learning
- The retention of manual flying skills in the automated cockpit
- An Antidote for Wandering Minds
- The Decoupled Mind: Mind-wandering Disrupts Cortical Phase-locking to Perceptual Events
- The Middle Way: Finding the Balance between Mindfulness and Mind-Wandering
- Insights from Quiet Minds: The Converging Fields of Mindfulness and Mind-Wandering
- Thoughts in Flight: Automation Use and Pilots’ Task-Related and Task-Unrelated Thought
- Window to the Wandering Mind: Pupillometry of Spontaneous Thought While Reading
- The default modes of reading: Modulation of posterior cingulate and medial prefrontal cortex connectivity associated with subjective and objective differences in reading experience
- The silver lining of a mind in the clouds: Interesting musings are associated with positive mood while mind-wandering
- Unnoticed intrusions: Dissociations of meta-consciousness in thought suppression
- Thinking one thing, saying another: The behavioral correlates of mind-wandering while reading aloud
- Mindfulness Training Improves Working Memory Capacity and GRE Performance While Reducing Mind Wandering.
- Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought
- Disentangling Decoupling: Comment on Smallwood
- Mindfulness and mind-wandering: Finding convergence through opposing constructs
- Inspired by Distraction Mind Wandering Facilitates Creative Incubation
- Insulation for daydreams: a role for tonic norepinephrine in the facilitation of internally guided thought
- The role of mind-wandering in measurements of general aptitude.
- Cooperation between the default mode network and the frontal–parietal network in the production of an internal train of thought
- Back to the future: Autobiographical planning and the functionality of mind-wandering
- Pupillometric Evidence for the Decoupling of Attention from Perceptual Input during Offline Thought
- Medicine for the wandering mind: Mind wandering in medical practice
- Meta-awareness, perceptual decoupling and the wandering mind
- Threatened to distraction: Mind-wandering as a consequence of stereotype threat
- Catching the mind in flight: Using behavioral indices to detect mindless reading in real time.
- Self-reflection and the temporal focus of the wandering mind.
- Out for a smoke: The impact of cigarette craving on zoning out while reading
- Slow fluctuations in attentional control of sensory cortex
- Eye movements during mindless reading.
- Mind-Wandering
- Experience sampling during fMRI reveals default network and executive system contributions to mind wandering
- Lost in the Sauce The effects of alcohol on mind wandering
- When attention matters: the curious incident of the wandering mind
- Going AWOL in the brain: mind wandering reduces cortical analysis of external events
- Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance
- The lights are on but no one’s home- the decoupling of executive resources when the mind-wanders
- Mind-wandering with and without awareness: An fMRI study of spontaneous thought processes
- The restless mind
- Zoning out while reading: Evidence for dissociations between experience and metaconsciousness.
- Pushing the Limits: Cognitive, Affective, & Neural Plasticity Revealed by an Intensive Multifaceted Intervention
- Early event-related brain potentials and hemispheric asymmetries reveal mind-wandering while reading and predict comprehension
- Language facilitates introspection: verbal mind-wandering has privileged access to consciousness
- Mindfulness in education: Enhancing academic achievement and student well-being by reducing mind-wandering
- Can I get me out of my head? Exploring strategies for controlling the self-referential aspects of the mind-wandering state during reading
- Meditation training influences mind wandering and mindless reading.
- Mind wandering minimizes mind numbing: Reducing semantic-satiation effects through absorptive lapses of attention.
- Mind-wandering and meta-awareness in hypnosis and meditation: Relating executive function across states of consciousness.
- Mind wandering “Ahas” versus mindful reasoning: alternative routes to creative solutions
- Mind Wandering While Driving What Does it Mean and What do we do about it?
- Motivating meta-awareness of mind wandering: A way to catch the mind in flight?
- The Richness of Inner Experience: Relating Styles of Daydreaming to Creative Processes.
- The science of mind wandering: empirically navigating the stream of consciousness
- Stimulating minds to wander
- Vigilance impossible: diligence, distraction, and daydreaming all lead to failures in a practical monitoring task
- Tracking Distraction: The Relationship Between Mind-Wandering, Meta-Awareness, and ADHD Symptomatology
- Young & restless: Validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth
- States of mind: Characterizing the neural bases of focus and mind-wandering through dynamic functional connectivity
Researchers

Jonathan Schooler
My lab’s research takes a “big picture” perspective in attempting to understand the nature of mental life, and in particular consciousness. Combining empirical, philosophical, and contemplative traditions, we address broad questions that cross traditional disciplinary boundaries.


James Elliott
James Elliott, Ph.D, is a cognitive neuroscientist with a background in behavioral, EEG, and fMRI methodologies. He has a keen interest in exploring how traditional meditation techniques can be used to help inform a scientific understanding of consciousness.

Michael Mrazek
Michael Mrazek, Ph.D. is the director of research at the University of California's Center for Mindfulness & Human Potential. His research identifies innovative ways to increase the effectiveness of mindfulness training, particularly in high schools. He also tests the limits of how much a person can improve through intensive evidence-based training programs that target health, mindfulness, and self-control.

Madeleine Gross
Madeleine studies the psychological basis of creative idea generation and insight. Using eye tracking technology, she also investigates how inter-individual differences in eye movement behavior may relate to dopamine-related cognition and personality traits, such as curiosity, schizotypy, and creativity.

Alissa Mrazek
Alissa Mrazek is a Research Assistant Professor in the Department of Psychology at UT Austin as well as a long-time collaborator with the META Lab. Alissa conducted a post-doctoral fellowship at the Center for Mindfulness and Human Potential with Dr. Jonathan Schooler from 2016-2020. Before working at UCSB, Alissa completed her Ph.D. in 2016 at Northwestern University where she began appreciating the synergistic benefits of integrative interventions—particularly when combining mindset training with strategy training.

Claire Zedelius
One line of Claire's research focuses on the role meta-awareness plays in the dynamic changes between states of mind wandering and focused attention. Another line examines the relationship between different types of mind wandering, creativity and curiosity.

Dharma Lewis
Dharma is a Mexico City native who is passionate about education and outreach. She earned her Biopsychology B.S. at UCSB where she studied the link between mindfulness, growth mindset, and mind-wandering as META Lab Manager. Her work currently focuses on pedagogical implications of meta-cognition, and the role of culture and mindsets in mindfulness.

Anusha Garg
Anusha studies the mechanisms and content of mind wandering. In the past, she's worked on assessing the content of the spontaneous stream of consciousness. She's currently investigating the differences between the quality of thoughts obtained during think aloud and silent mind wandering protocols.

Shivang Shelat
Shivang's interests lie in interactions between mind-wandering, memory, and attentional capture. He also uses principles in attention neuroscience to better understand human-computer interactions. He is co-advised by Barry Giesbrecht.

My name is Jinny Kim, and I am a 3rd year Biopsychology major and Applied Psychology minor. I am assisting James Elliott regarding fluctuations of experience and EEG during meditation. My specific interests are dream analysis and psychotherapy for criminals.
Research Collaborators
Jonathan smallwood.

What is the Research on Daydreaming
Can You Focus Your Daydreaming

Dyslexic Advantage Mind Wandering with Dr Jonathan Schooler

How Is Day Dreaming Useful?

It Took You Three Attempts to Read That Simple Paragraph Here's Why 1

Mind Wandering and Meta-Awareness

Wegstock 18 Mind Wandering Jonathan Schooler

What is Day Dreaming?

What's the connection between daydreaming and ADHD 1

Who daydreams and what does it mean?
- U.S. Department of Education, Institute of Educational Science. (2011-2015) Mind-wandering During Reading

The Effectiveness Focusing And Switching Attention Training In Reducing Mind Wandering During E-Learning Among University Students
- Wesam Hamdy Elkasaby , Youssef Mohmed Shalaby
The current study aimed to identify the effectiveness of attention training (training on focusing and switching attention) in reducing mind wandering during e-learning among university students. The research sample consisted of (52) male and female university students who were randomly distributed equally among the experimental and control groups. The study used measures: mind wandering scale, attention training tasks including: Stroop task and Switching Attention Task ( SAT ).
The results of the study resulted in statistically significant differences at the level of significance (0.01) between the mean [1] scores of the experimental group and the control group in the post-measurement of mind wandering in favor of the control group. There are also statistically significant differences at the level of significance (0.01) between the means of the scores of the pre and post scores of the experimental group in mind wandering. In favor of pre-testing. This finding indicates the effectiveness of switching and focusing attention training in reducing mind wandering in the experimental group.
How to Cite
- Endnote/Zotero/Mendeley (RIS)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License .
CC Attribution-NonCommercial-NoDerivatives 4.0
Old publisher: Transnational Press London
New Publisher: Migration Letters & The London Publishers

- International edition
- Australia edition
- Europe edition

‘Like a film in my mind’: hyperphantasia and the quest to understand vivid imaginations
Research that aims to explain why some people experience intense visual imagery could lead to a better understanding of creativity and some mental disorders
W illiam Blake’s imagination is thought to have burned with such intensity that, when creating his great artworks, he needed little reference to the physical world. While drawing historical or mythical figures, for instance, he would wait until the “spirit” appeared in his mind’s eye. The visions were apparently so detailed that Blake could sketch as if a real person were sitting before him.
Like human models, these imaginary figures could sometimes act temperamentally. According to Blake biographer John Higgs , the artist could become frustrated when the object of his inner gaze casually changed posture or left the scene entirely. “I can’t go on, it is gone! I must wait till it returns,” Blake would declaim.
Such intense and detailed imaginations are thought to reflect a condition known as hyperphantasia, and it may not be nearly as rare as we once thought, with as many as one in 30 people reporting incredibly vivid mind’s eyes.
Just consider the experiences of Mats Holm, a Norwegian hyperphantasic living in Stockholm. “I can essentially zoom out and see the entire city around me, and I can fly around inside that map of it,” Holm tells me. “I have a second space in my mind where I can create any location.”
This once neglected form of neurodiversity is now a topic of scientific study, which could lead to insights into everything from creative inspiration to mental illnesses such as post-traumatic stress disorder and psychosis.
Francis Galton – better known as a racist and the “father of eugenics” – was the first scientist to recognise the enormous variation in people’s visual imagery. In 1880, he asked participants to rate the “illumination, definition and colouring of your breakfast table as you sat down to it this morning”. Some people reported being completely unable to produce an image in the mind’s eye, while others – including his cousin Charles Darwin – could picture it extraordinarily clearly.
“Some objects quite defined. A slice of cold beef, some grapes and a pear, the state of my plate when I had finished and a few other objects are as distinct as if I had photos before me,” Darwin wrote to Galton.
Unfortunately, Galton’s findings failed to fire the imagination of scientists at the time. “The psychology of visual imagery was a very big topic, but the existence of people at the extremes somehow disappeared from view,” says Prof Adam Zeman at Exeter University. It would take more than a century for psychologists such as Zeman to take up where Galton left off.

Even then, much of the initial research focused on the poorer end of the spectrum – people with aphantasia , who claim to lack a mind’s eye. Within the past five years, however, interest in hyperphantasia has started to grow, and it is now a thriving area of research.
To identify where people lie on the spectrum, researchers often use the Vividness of Visual Imagery Questionnaire (VVIQ), which asks participants to visualise a series of 16 scenarios, such as “the sun rising above the horizon into a hazy sky” and then report on the level of detail that they “see” in a five-point scale. You can try it for yourself. When you picture that sunrise, which of the following statements best describes your experience?
1. No image at all, you only “know” that you are thinking of the object 2. Vague and dim 3. Moderately clear and lively 4. Clear and reasonably vivid 5. Perfectly clear and as vivid as real seeing
The final score is the sum of all 16 responses, with a maximum of 80 points. In large surveys, most people score around 55 to 60 . Around 1% score just 16; they are considered to have extreme aphantasia; 3%, meanwhile, achieve a perfect score of 80, which is extreme hyperphantasia.
The VVIQ is a relatively blunt tool, but Reshanne Reeder, a lecturer at Liverpool University, has now conducted a series of in-depth interviews with hyperphantasic people – research that helps to delineate the peculiarities of their inner lives. “As you talk to them, you start to realise that this is a very different experience from most people’s experience,” she says. “It’s extremely immersive, and their imagery affects them very emotionally.”
Some people with hyperphantasia are able to merge their mental imagery with their view of the world around them. Reeder asked participants to hold out a hand and then imagine an apple sitting in their palm. Most people feel that the scene in front of their eyes is distinct from that inside their heads. “But a lot of people with hyperphantasia – about 75% – can actually see an apple in the hand in front of them. And they can even feel its weight.”
As you might expect, these visual abilities can influence career choices. “Aphantasia does seem to bias people to work in sciences, maths or IT – those Stem professions – whereas hyperphantasia nudges people to work in what are traditionally called creative professions,” says Zeman. “Though there are many exceptions.”

Reeder recalls one participant who uses her hyperphantasia to fuel her writing. “She said she doesn’t even have to think about the stories that she’s writing, because she can see the characters right in front of her, acting out their parts,” Reeder recalls.
H yperphantasia can also affect people’s consumption of art. Novels, for example, become a cinematic experience. “For me, the story is like a film in my mind,” says Geraldine van Heemstra , an artist based in London. Holm offers the same description. “When I listen to an audiobook, I’m running a movie in my head.”
This is not always an advantage. Laura Lewis Alvarado, a union worker who is also based in London, describes her disappointment at watching The Golden Compass, the film adaptation of the first part of Philip Pullman’s His Dark Materials . “I already had such a clear idea of how every character looked and acted,” she says. The director’s choices simply couldn’t match up.
Zeman’s research suggests that people with hyperphantasia enjoy especially rich autobiographical memories. This certainly rings true for Van Heemstra. When thinking of trips in the countryside, she can recall every step of her walks, including seemingly inconsequential details. “I can picture even little things, like if I dropped something and picked it up,” she says.
after newsletter promotion
Exactly where these abilities come from is unknown. Aphantasia is known to run in families, so it’s reasonable to expect that hyperphantasia may be the same. Like many other psychological traits, our imaginative abilities probably come from a combination of nature and nurture, which will together shape the brain’s development from infancy to old age.
Zeman has taken the first steps to investigate the neurological differences that underpin the striking variation in the mind’s eye. Using fMRI to scan the brains of people at rest, he has found that hyperphantasic people have greater connectivity between the prefrontal cortex, which is involved in “higher-order” thinking such as planning and decision-making, and the areas responsible for visual processing, which lie towards the back of the skull.
“My guess is that if you say ‘apple’ to somebody with hyperphantasia, the linguistic representation of ‘apple’ in the brain immediately transmits the information to the visual system,” says Zeman. “For someone with aphantasia, the word and concept of ‘apple’ operate independently of the visual system, because those connections are weaker.”
Further research will no doubt reveal the nuances in this process. Detailed questionnaires by Prof Liana Palermo at the Magna Graecia University in Catanzaro, Italy, for instance, suggest that there may be two subtypes of vivid imagery . The first is object hyperphantasia, which, as the name suggests, involves the capacity to imagine items in extreme detail.
The second is spatial hyperphantasia, which involves an enhanced ability to picture the orientation of different items relative to one another and perform mental rotations. “They also report a heightened sense of direction,” Palermo says. This would seem to match Holm’s descriptions of the detailed 3D cityscape that allows him to find a route between any two locations.

Many mysteries remain. A large survey by Prof Ilona Kovács, at Eötvös Loránd University in Hungary, suggests that hyperphantasia is far more common among children, and fades across adolescence and into adulthood. She suspects that this may reflect differences in how the brain encodes information. In infancy, our brains store more sensory details, which are slowly replaced by more abstract ideas. “The child’s memories offer a more concrete appreciation of the world,” she says – and it seems that only a small percentage of people can maintain this into later life.
Reeder, meanwhile, is interested in studying the consequences of hyperphantasia for mental health. It is easy to imagine how vivid memories of upsetting events could worsen the symptoms of anxiety or post-traumatic stress disorder, for example.
Reeder is also investigating the ways that people’s mental imagery may influence the symptoms of illnesses such as schizophrenia . She suspects that, if someone is already at risk of psychosis, then hyperphantasia may lead them to experience vivid hallucinations, while aphantasia may increase the risk of non-sensory delusions, such as fears of persecution.
For the moment, this remains an intriguing hypothesis, but Reeder has shown that people with more vivid imagery in daily life are also more susceptible to seeing harmless “ pseudo-hallucinations ” in the laboratory. She asked participants to sit in a darkened room while watching a flickering light on a screen – a set-up that gently stimulates the brain’s visual system. After a few minutes, many people will start to see simple illusions, such as geometric shapes. People with higher VVIQ scores, however, tended to see far more complex scenes – such as a stormy beach, a medieval castle or a volcano. “It was quite psychedelic,” says Lewis Alvarado, who took part in the experiment.
Reeder emphasises that the participants in her study were perfectly able to recognise that these pseudo-hallucinations were figments of their imagination. “If someone never has reality discrimination issues, then I don’t think they’re going to be more prone to psychosis.” For those with mental illness, however, a better understanding of the mind’s eye could offer insights into the patient’s experiences.
For now, Reeder hopes that greater awareness of hyperphantasia will help people to make the most of their abilities. “It’s a skill that could be tapped,” she suggests.
Many of the people I have interviewed are certainly grateful to know a little more about the mind’s eye and the way theirs differs from the average person’s.
Lewis Alvarado, for instance, only came across the term when she was listening to a podcast about William Blake, which eventually led her to contact Reeder. “For the first month or so I couldn’t get it out of my head,” she says. “It’s not something I talk about loads, but I think it has helped me to realise why I experience things more intensely, which is comforting.”
David Robson is the author of The Laws of Connection: 13 Social Strategies That Will Transform Your Life , published by Canongate on 6 June (£18.99). To support the Guardian and Observer , order your copy at guardianbookshop.com . Delivery charges may apply
- The Observer
- Neuroscience
- Neurodiversity

Keep winning at tennis? You may see more images each second, scientists say

VR goggles for mice create immersive scenarios for brain research

Goalkeepers perceive the world differently, study suggests
Science Weekly Could we end migraines for good? – podcast
Science Weekly Has a 25-year-old bet taken us a step closer to understanding consciousness? – podcast

Short daytime naps may keep brain healthy as it ages, study says

Paralysed man walks using device that reconnects brain with muscles
Scientists discover brain signals for chronic pain

Talking to babies may help shape brain structure, research finds

Science Weekly Will psychedelic drugs transform mental health treatment? – podcast
Most viewed.

The Palgrave Encyclopedia of the Possible pp 868–875 Cite as
Mind Wandering
- David D. Preiss 2
- Reference work entry
- First Online: 01 January 2023
80 Accesses
Mind wandering plays a significant role in the psychological construction of possible worlds. It has a functional connection with pretend play, autobiographical planning, and creativity. Because of its association with pretend play, mind wandering is a precursor of creativity and imagination. Additionally, mind wandering is involved in the preparation for alternative futures during autobiographical planning. Thus, it has a strong connection with autonoetic awareness and episodic memory. Mind wandering is also related to idea generation during the incubation stage of the creative process. Moreover, during long-term endeavors, creative individuals adopt a disposition of mindful mind wandering. By advancing research on mind wandering, we will gain more knowledge about the multiple ways human beings transcend their current experience, in addition to open new inquiries about the relationship between consciousness, memory, and creativity.
Author’s note. Work on this encyclopedia entry was supported by grant FONDECYT 1181095 from ANID. Corresponding author at: Escuela de Psicología, Pontificia Universidad Católica de Chile, Av Vicuña Mackenna 4860, Macul, Santiago 7820436, Chile.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Antrobus, J. S. (1968). Information theory and stimulus-independent thought. British Journal of Psychology, 59 (4), 423–430. https://doi.org/10.1111/j.2044-8295.1968.tb01157.x .
Article Google Scholar
Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W. Y., Franklin, M. S., & Schooler, J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23 (10), 1117–1122. https://doi.org/10.1177/0956797612446024 .
Baird, B., Smallwood, J., & Schooler, J. W. (2011). Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20 (4), 1604–1611. https://doi.org/10.1016/j.concog.2011.08.007 .
Baumeister, R. F., & Masicampo, E. J. (2010). Conscious thought is for facilitating social and cultural interactions: How mental simulations serve the animal-culture interface. Psychological Review, 117 (3), 945–971. https://doi.org/10.1037/a0019393 .
Beaty, R. E., & Schacter, D. L. (2017). Creativity, self-generated thought, and the brain’s default network. In M. Karwowski & J. C. Kaufman (Eds.), The creative self: Effect of beliefs, self-efficacy, mindset, and identity (pp. 171–183). Academic Press. https://doi.org/10.1016/B978-0-12-809790-8.00010-8 .
Chapter Google Scholar
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124 (1), 1–38. https://doi.org/10.1196/annals.1440.011 .
Cosmelli, D., & Preiss, D. D. (2014). On the temporality of creative insight: A psychological and phenomenological perspective. Frontiers in Psychology, 5 (1184), 1–6. https://doi.org/10.3389/fpsyg.2014.01184 .
Csikszentmihalyi, M. (1996). Creativity: Flow and the psychology of discovery and invention . HarperCollins Publishers.
Google Scholar
Fein, G. (1987). Pretend play: Creativity and consciousness. In P. Gorlitz & J. Wohlwill (Eds.), Curiosity, imagination and play (pp. 281–304). Lawrence Erlbaum Associates.
Gardner, H. (1993). Creating minds: An anatomy of creativity seen through the lives of Freud, Einstein, Picasso, Stravinsky, Eliot, Graham, and Gandhi . Basic Books.
Giambra, L. M. (1989). Task-unrelated-thought frequency as a function of age: A laboratory study. Psychology and Aging, 4 (2), 136–143. https://doi.org/10.1037/0882-7974.4.2.136 .
Gruber, H. E., & Wallace, D. B. (2001). Creative work: The case of Charles Darwin. American Psychologist, 56 (4), 346–349. https://doi.org/10.1037/0003-066X.56.4.346 .
Hass, R. W., & Weisberg, R. W. (2015). Revisiting the 10-year rule for composers from the great American songbook: On the validity of two measures of creative production. Psychology of Aesthetics, Creativity, and the Arts, 9 (4), 471–479. https://doi.org/10.1037/aca0000021 .
Hoffmann, J., & Russ, S. (2012). Pretend play, creativity, and emotion regulation in children. Psychology of Aesthetics, Creativity, and the Arts, 6 (2), 175–184. https://doi.org/10.1037/a0026299 .
Immordino-Yang, M. H., Christodoulou, J. A., & Singh, V. (2012). Rest is not idleness: Implications of the brain’s default mode for human development and education. Perspectives on Psychological Science, 7 (4), 352–364. https://doi.org/10.1177/1745691612447308 .
Kane, M. J., Brown, L. H., McVay, J. C., Silvia, P. J., Myin-Germeys, I., & Kwapil, T. R. (2007). For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science, 18 (7), 614–621. https://doi.org/10.1111/j.1467-9280.2007.01948.x .
Killingsworth, M. A., & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science, 330 (6006), 932. https://doi.org/10.1126/science.1192439 .
Lee, A. W., & Russ, S. W. (2018). Pretend play, divergent thinking, and self-perceptions of creativity: A longitudinal study. The International Journal of Creativity & Problem Solving, 28 (1), 73–88.
Lindquist, S. I., & McLean, J. P. (2011). Daydreaming and its correlates in an educational environment. Learning and Individual Differences, 21 (2), 158–167. https://doi.org/10.1016/j.lindif.2010.12.006 .
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., & Macrae, C. N. (2007). Wandering minds: The default network and stimulus-independent thought. Science, 315 (5810), 393–395. https://doi.org/10.1126/science.1131295 .
McMillan, R. L., Kaufman, S. B., & Singer, J. L. (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4 (626), 1–9. https://doi.org/10.3389/fpsyg.2013.00626 .
McVay, J. C., Kane, M. J., & Kwapil, T. R. (2009). Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review, 16 (5), 857–863. https://doi.org/10.3758/PBR.16.5.857 .
Moore, M., & Russ, S. W. (2006). Pretend play as a resource for children: Implications for pediatricians and health professionals. Journal of Developmental and Behavioral Pediatrics, 27 (3), 237–248. https://doi.org/10.1097/00004703-200606000-00011 .
Mrazek, M. D., Smallwood, J., Franklin, M. S., Chin, J. M., Baird, B., & Schooler, J. W. (2012). The role of mind-wandering in measurements of general aptitude. Journal of Experimental Psychology: General, 141 (4), 788–798. https://doi.org/10.1037/a0027968 .
Preiss, D. D., & Cosmelli, D. (2017). Mind wandering, creative writing, and the self. In M. Karwowski & J. C. Kaufman (Eds.), The creative self: Effect of beliefs, self-efficacy, mindset, and identity (pp. 301–313). Academic Press. https://doi.org/10.1016/B978-0-12-809790-8.00017-0 .
Preiss, D. D., Cosmelli, D., Grau, V., & Ortiz, D. (2016). Examining the influence of mind wandering and metacognition on creativity in university and vocational students. Learning and Individual Differences, 51 , 417–426. https://doi.org/10.1016/j.lindif.2016.07.010 .
Preiss, D. D., Ibaceta, M., Ortiz, D., Carvacho, H., & Grau, V. (2019). An exploratory study on mind wandering, metacognition, and verbal creativity in Chilean high school students. Frontiers in Psychology, 10(1118), 1–6. https://doi.org/10.3389/fpsyg.2019.01118 .
Preiss, D. D., Lynch, S. F., McKay, A. S., & Kaufman, J. C. (2020). Poetry. In S. Pritzker & M. Runco (Eds.), Encyclopedia of creativity (3rd ed., pp. 367–370). Academic Press. https://doi.org/10.1016/B978-0-12-809324-5.23842-X .
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98 (2), 676–682. https://doi.org/10.1073/pnas.98.2.676 .
Raichle, M. E., & Snyder, A. Z. (2007). A default mode of brain function: A brief history of an evolving idea. NeuroImage, 37 (4), 1083–1090. https://doi.org/10.1016/j.neuroimage.2007.02.041 .
Roby, A. C., & Kidd, E. (2008). The referential communication skills of children with imaginary companions. Developmental Science, 11 (4), 531–540. https://doi.org/10.1111/j.1467-7687.2008.00699.x .
Russ, S. W. (2020). Mind wandering, fantasy, and pretend play. In D. D. Preiss, D. Cosmelli, & J. K. Kaufman (Eds.), Creativity and the wandering mind (pp. 231–248). Academic Press. https://doi.org/10.1016/B978-0-12-816400-6.00010-9 .
Russ, S. W., Robins, A. L., & Christiano, B. A. (1999). Pretend play: Longitudinal prediction of creativity and affect in fantasy in children. Creativity Research Journal, 12 (2), 129–139. https://doi.org/10.1207/s15326934crj1202_5 .
Schacter, D. L., Addis, D. R., & Buckner, R. L. (2007). Remembering the past to imagine the future: The prospective brain. Nature Reviews Neuroscience, 8 , 657–661. https://doi.org/10.1038/nrn2213 .
Seli, P., Carriere, J. S. A., & Smilek, D. (2015). Not all mind wandering is created equal: Dissociating deliberate from spontaneous mind wandering. Psychological Research, 79 (5), 750–758. https://doi.org/10.1007/s00426-014-0617-x .
Seli, P., Risko, E. F., & Smilek, D. (2016). Assessing the associations among trait and state levels of deliberate and spontaneous mind wandering. Consciousness and Cognition, 41 , 50–56. https://doi.org/10.1016/j.concog.2016.02.002 .
Singer, J. L. (1974). Daydreaming and the stream of thought: Daydreams have usually been associated with idleness and inattentiveness. Now, however, through an empirical research program, their general function and adaptive possibilities are being elucidated. American Scientist, 62 , 417–425.
Singer, J. L. (1975). Navigating the stream of consciousness: Research in daydreaming and related inner experience. American Psychologist, 30 (7), 727–738. https://doi.org/10.1037/h0076928 .
Sio, U. N., & Ormerod, T. C. (2009). Does incubation enhance problem solving? A meta-analytic review. Psychological Bulletin, 135 (1), 94–120. https://doi.org/10.1037/a0014212 .
Smallwood, J. (2013). Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychological Bulletin, 139 (3), 519–535. https://doi.org/10.1037/a0030010 .
Smallwood, J., Fishman, D. J., & Schooler, J. W. (2007). Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin and Review, 14 (2), 230–236. https://doi.org/10.3758/BF03194057 .
Smallwood, J., McSpadden, M., & Schooler, J. W. (2008). When attention matters: The curious incident of the wandering mind. Memory & Cognition, 36 (6), 1144–1150. https://doi.org/10.3758/MC.36.6.1144 .
Smallwood, J., Nind, L., & O’Connor, R. C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition, 18 (1), 118–125. https://doi.org/10.1016/j.concog.2008.11.004 .
Smallwood, J., & O’Connor, R. C. (2011). Imprisoned by the past: Unhappy moods lead to a retrospective bias to mind wandering. Cognition and Emotion, 25 (8), 1481–1490. https://doi.org/10.1080/02699931.2010.545263 .
Smallwood, J., Ruby, F. J. M., & Singer, T. (2013). Letting go of the present: Mind-wandering is associated with reduced delay discounting. Consciousness and Cognition, 22 (1), 1–7. https://doi.org/10.1016/j.concog.2012.10.007 .
Smallwood, J., & Schooler, J. W. (2006). The restless mind. Psychological Bulletin, 132 (6), 946–958. https://doi.org/10.1037/0033-2909.132.6.946 .
Smallwood, J., & Schooler, J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66 (1), 487–518. https://doi.org/10.1146/annurev-psych-010814-015331 .
Sutherland, S. L., & Friedman, O. (2013). Just pretending can be really learning: Children use pretend play as a source for acquiring generic knowledge. Developmental Psychology, 49 (9), 1660–1668. https://doi.org/10.1037/a0030788 .
Szpunar, K. K., Moulton, S. T., & Schacter, D. L. (2013). Mind wandering and education: From the classroom to online learning. Frontiers in Psychology, 4 (495), 1–7. https://doi.org/10.3389/fpsyg.2013.00495 .
Takeuchi, H., Taki, Y., Hashizume, H., Sassa, Y., Nagase, T., Nouchi, R., & Kawashima, R. (2011). Cerebral blood flow during rest associates with general intelligence and creativity. PLoS One, 6 (9), e25532. https://doi.org/10.1371/journal.pone.0025532 .
Tulving, E. (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53 , 1–25. https://doi.org/10.1146/annurev.psych.53.100901.135114 .
Wallace, C. E., & Russ, S. W. (2015). Pretend play, divergent thinking, and math achievement in girls: A longitudinal study. Psychology of Aesthetics, Creativity, and the Arts, 9 (3), 296–305. https://doi.org/10.1037/a0039006 .
Whitebread, D., & O’Sullivan, L. (2020). Pretend play in young children and the emergence of creativity. In D. D. Preiss, D. Cosmelli, & J. C. Kaufman (Eds.), Creativity and the wandering mind (pp. 205–230). Academic Press. https://doi.org/10.1016/B978-0-12-816400-6.00009-2 .
Woolley, J. D., Bunce, L., & Boerger, E. A. (2020). Relations between imagination and creativity. In D. D. Preiss, D. Cosmelli, & J. C. Kaufman (Eds.), Creativity and the wandering mind (pp. 181–203). Academic Press. https://doi.org/10.1016/B978-0-12-816400-6.00008-0 .
Download references
Author information
Authors and affiliations.
Pontificia Universidad Católica de Chile, Santiago, Chile
David D. Preiss
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to David D. Preiss .
Editor information
Editors and affiliations.
Dublin City University, Dublin, Ireland
Vlad Petre Glăveanu
Section Editor information
Paris Descartes University, Paris, France
Samira Bourgeois-Bougrine
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry.
Preiss, D.D. (2022). Mind Wandering. In: Glăveanu, V.P. (eds) The Palgrave Encyclopedia of the Possible. Palgrave Macmillan, Cham. https://doi.org/10.1007/978-3-030-90913-0_16
Download citation
DOI : https://doi.org/10.1007/978-3-030-90913-0_16
Published : 26 January 2023
Publisher Name : Palgrave Macmillan, Cham
Print ISBN : 978-3-030-90912-3
Online ISBN : 978-3-030-90913-0
eBook Packages : Behavioral Science and Psychology Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Spending 30 years in a psychological study by Jack and Jeanne Block warped journalist Susannah Breslin's life
News ticker.
ASIO chief Mike Burgess and AFP Commissioner Reece Kershaw are addressing the National Press Club
For three decades, Susannah Breslin was studied by researchers.
As a baby, her parents enrolled her in a University of California, Berkeley laboratory preschool, a place that was "essentially designed for spying on children", Breslin tells ABC RN's All in the Mind .
There, she unknowingly became the subject of a study that would continue well into her adult life.
"My parents were intellectuals. They were cool in temperament. They were not touchy-feely. And they, I think, had high expectations for their children," Breslin says.
The preschool was exclusive, with a long waitlist, and her parents "thought it would be cool to have me enrolled [and] involved in something important", she says.
They thought they were setting their daughter off on a path of exceptionalism.
"My principal investigators were benevolent; they wanted to enlighten humanity. They were sort of working in service of the greater good. That's why they were collecting our data. That's why they were studying us," Breslin says.
"And the cost was we didn't have a private life of our own."
'Somebody on the other side of the mirror'
In the 1960s, American psychologists Jack and Jeanne Block developed a longitudinal study to observe how personality traits and cognition develop over the course of a lifetime.
"The only way to find [that] out was to gather together a group of kids and study them from childhood and into adulthood," Breslin explains.
She was one of 128 children recruited.
One of her earliest memories of the preschool is being in a room with activities like puzzles and toy animals, playing games with an adult she later learned was testing and studying her, and observing her interactions with other children.
Once she'd left the preschool for primary school, Breslin was routinely brought back to the university at key developmental stages, where researchers would capture data from IQ, personality and other tests.
They would assess her report cards. They would ask her about her life.
During one of these sessions, when she was seven years old, Breslin recalls an incredible moment.
She was in a room with a researcher who had placed a bowl of lollies between the two of them.
"The examiner was asking me all these questions about myself and then at one point he said, 'Would you like some candy?'"
The young Breslin wondered if this might be a kind of test, so she refused the offer. Soon after, the examiner said he needed to leave for a minute but would be right back.
As soon as he walked out of the room, Breslin jumped across the table and lunged for the bowl, accidentally knocking it over in the process.
"I started grabbing the candy and shoving it in my mouth, hoping I wasn't going to get in trouble for making a mess," she says.
"And then for some reason I looked — I remember this very distinctly — into this mirror that was on the wall. And I could see and feel my cheeks turning pink, and I sensed that there was somebody on the other side of the mirror watching me.
"And in fact, that was a one-way mirror, and somebody was on the other side, spying on me."
Study 'like a third parent'
When Breslin got older and found out about the study, she was in two minds about the realisation she was being observed.
"On the one hand, I liked it. My parents were distant, they were busy, they were preoccupied with their careers … and their marriage was falling apart.
"When I was in an experiment room at the university, it was exactly what I wanted. I was the centre of attention. And they were interested in nothing but me."
The idea that Breslin was important, that she mattered and that someone cared were not messages she was getting at home, she says.
"At the same time, I think I felt a lot of pressure to perform at some high level; that I was supposed to prove to [the researchers] that I was exceptional. And if I didn't, maybe I wouldn't be able to come back again."
The study "was kind of like a third parent", she says.
Researchers served as confidants. Breslin told them more secrets than she told her parents.
"The researchers were interested in the things that I was going through. They were interested in whether or not we were using drugs, they were interested in how our parents' divorces were affecting us. And I told them things that I didn't tell anyone else.
"I have this idea that this study potentially knew me better than I knew myself."
Humans more than a 'mathematical equation'
Today Breslin is an investigative journalist, and recently wrote a book about her experience being studied, Data Baby: My Life in a Psychological Experiment.
The process of writing that helped her to understand just how much she divulged over the course of the study.
"You kind of surrender your life story to somebody else … I was unsure if I was the author of the story of my life, or if someone else was," she says.
"In my opinion, my principal investigators believed that a person was the sum total of their data; that a human being was the answer to a mathematical equation, and if you could just make the right calculation or perform the right analysis or extract the correct amount of data, you could understand that person.
"And I think that is wrong. I think that there is something about people that goes beyond that; that there is a certain essential nature to people that is impossible to quantify."
Yet, Breslin credits the study for her life today.
"I have struggled with anxiety and depression my whole life. And I do wonder if I hadn't been studied and I hadn't had [it] in my life, that the outcome for me might have been much bleaker; that I might have killed myself or gotten involved in something dangerous that I couldn't get myself out of.
"So while it was certainly complicated to be studied, I think ultimately it rescued me from a worse fate."
Breslin's final contact with the researchers was in 1999, at the age of 32.
Since then, she says she's been forced "to construct my own narrative and decide for myself who I am, rather than entrusting that task to somebody else".
RN in your inbox
Get more stories that go beyond the news cycle with our weekly newsletter.
- X (formerly Twitter)
- Child Health and Behaviour
- Community and Society
- Family and Relationships
- Human Interest
- Science and Technology
- United States
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
5 things to keep in mind when you hear about Gen Z, Millennials, Boomers and other generations
Generation Z. Millennials. Baby Boomers. It’s hard not to run into eye-catching headlines about generations these days. And it’s easy to feel like many of these headlines are just clickbait, all fluff and no substance. But is that really the case?
At Pew Research Center, we think it can be useful to talk about generations. But there are some important considerations for readers to keep in mind whenever they come across a news story or research about generations:
Generational categories are not scientifically defined. The boundaries that place one person in Gen Z and another in the Millennial generation are not precise, definitive or universally agreed on. Even the names of generations are not uniformly adopted: Is it Millennials or Generation Y ? Gen Z or iGen ?
People born near the boundaries of these generational groupings can feel particularly uncomfortable being lumped in with those much older or younger than them, and for good reason. The media and researchers – Pew Research Center included – have not always been as clear as we should that generational boundaries are not a hard science.
Generational labels can lead to stereotypes and oversimplification. All Millennials or Baby Boomers are not the same, just as all Southerners, all Catholics or all Black Americans are not the same. Shared experiences and identities should be recognized – and at their best can even be empowering – but this shouldn’t come at the expense of individuality.
Discussions about generation often focus on differences instead of similarities. Conflict tends to get more attention than consensus. So watch out for news stories or research articles that assume or exaggerate intergenerational divides that may actually be quite small. “ OK Boomer ” became a cultural meme, but it probably overstates the divide between younger and older generations. After all, most of us have some combination of parents, grandparents, kids and grandkids we love, making our family lives interconnected.
Conventional views of generations can carry an upper-class bias. Popular history recalls that Baby Boomers in the 1960s and ’70s were deeply opposed to the Vietnam War. This notion is based on attention-grabbing protests on college campuses and at political events. But many high-quality surveys at the time showed that younger Americans – most of whom were not attending college – were more supportive of the war than older generations who had lived through previous conflicts. Readers today should similarly question whether stereotypes of Gen Z might be skewed toward the experiences of the upper middle class.
People change over time. It’s worth pausing when you hear someone say that “kids today” are so different from their predecessors. Young adults have always faced a different environment than their parents, and it’s common for their elders to express some degree of concern or alarm. (“Why is his hair dyed green?”)
Don’t assume that what you see today is what you’ll get tomorrow. People change as they grow older, pursue careers and form families. Gen Zers will no doubt walk differently in the world by 2050, just as today’s Baby Boomers are different from their younger selves. Generational signals can sometimes be lasting, but youth itself is not a permanent state.
So is it all just hype?
If you’ve read this far, your suspicions about generational labels may have hardened. That’s OK. Our recommendation is for readers to bring a healthy dose of skepticism to the generational discussions they see. Readers should also hold media and research organizations that focus on generations – including Pew Research Center – to a high standard.
Despite these cautions, we still believe generational thinking can help us understand how societies change over time. The eras in which we come of age can leave a signature of common experiences and perspectives. Events such as terrorist attacks, wars, recessions and pandemics can shape the opportunities and mindsets of those most affected by them.
Similarly, historical advances like desegregation, effective birth control, the invention of the internet and the arrival of artificial intelligence can fundamentally change how people live their lives, and the youngest generations are often in the vanguard. At the same time, some events can affect people across generations, moving everyone in one direction or another.
It’s wise to think of terms like Gen Z, Millennial, Gen X and Baby Boomer as general reference points instead of scientific facts. At Pew Research Center, we’ll continue to use these and other labels to help our readers navigate a changing world. But we’ll do so sparingly – and only when the data supports the use of the generational lens .
- Age & Generations
- Demographic Research
- Generation X
- Generation Z
- Generations
- Greatest Generation
- Methodological Research
- Millennials
- Silent Generation

How Teens and Parents Approach Screen Time
Who are you the art and science of measuring identity, u.s. centenarian population is projected to quadruple over the next 30 years, older workers are growing in number and earning higher wages, teens, social media and technology 2023, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
- Search Menu
- Volume 2024, Issue 1, 2024 (In Progress)
- Volume 2023, Issue 1, 2023
- Special Issues
- Author Guidelines
- Journal Policies
- Submission Site
- Open Access
- Why publish with this journal?
- Call For Papers
- About Neuroscience of Consciousness
- About the Association for the Scientific Study of Consciousness
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, mind wandering, cognitive control, why the mind wanders, explanations, predictions, philosophical implications, acknowledgments.
- < Previous
Why does the mind wander?
- Article contents
- Figures & tables
- Supplementary Data
Joshua Shepherd, Why does the mind wander?, Neuroscience of Consciousness , Volume 2019, Issue 1, 2019, niz014, https://doi.org/10.1093/nc/niz014
- Permissions Icon Permissions
I seek an explanation for the etiology and the function of mind wandering episodes. My proposal—which I call the cognitive control proposal—is that mind wandering is a form of non-conscious guidance due to cognitive control. When the agent’s current goal is deemed insufficiently rewarding, the cognitive control system initiates a search for a new, more rewarding goal. This search is the process of unintentional mind wandering. After developing the proposal, and relating it to the literature on mind wandering and on cognitive control, I discuss explanations the proposal affords, testable predictions the proposal makes, and philosophical implications the proposal has.
Makes a novel and empirically tractable proposal regarding why the mind wanders
Offers novel explanations of data on mind wandering
Offers predictions for future work on mind wandering
Integrates literature on cognitive control with the literature on mind wandering
Discusses implications for a philosophical account of the nature of mind wandering
Minds wander
Some wander more than others, but human ones wander a lot. A much-cited estimate, due to Killingsworth and Gilbert (2010) , has it that the awake human mind spends from a third to half its time wandering. That’s a big range, a rough estimate, and there are good reasons to be suspicious of it (see Seli et al. 2018 ). The actual number will likely depend a bit upon the nature of mind wandering, a bit upon whether we have the right measure to produce such an estimate, and of course a bit on individual variability. Estimates aside, though, introspection reports that the mind wanders surprisingly often. My question here is this.
Why does it happen?
Sub-questions include the following. What drives the mind to wander? Does anything drive it to wander? Is the transition from focused thought to meandering thought random? Is it a failure of control, or is there some dark purpose behind these mental movements?
In the next section, I set the table by discussing a few interesting features of mind wandering, as well as a few recent proposals about its etiology, and its function. It is easy to conflate these two, since if mind wandering has a function its etiology may very well help illuminate it, but the questions are distinct. Here, I am more interested in why mind wandering happens—about its etiology. It turns out, though, that on my proposal mind wandering happens for good functional reasons. I develop this proposal, which I call the cognitive control proposal, in Cognitive control and Why the mind wanders sections. In Explanations section, I discuss some explanations this proposal makes possible. In Predictions section, I discuss some predictions that could confirm or disconfirm the proposal. In Philosophical implications section, I discuss implications for a philosophical account of the nature of mind wandering.
By referring to this phenomenon as mind wandering, a term familiar to the lay person, we hope to elevate the status of this research into mainstream psychological thinking (946).
As Murray et al. (2019) report, since that review, usage of “mind wandering” has risen dramatically. Only the Smallwood and Schooler paper used the term in a title or abstract in 2006. In 2018, the term appeared in 132 titles or abstracts.
Increased attention to the range of phenomena grouped together by “mind wandering” is salutary. But theorists recognize that the range of processes the term groups may contain multiple etiologies and processing signatures. Accordingly, theorists have proposed many sub-types of mind wandering, categorizing episodes of mind wandering in at least three distinct ways.
The first two involve a conception of mind wandering as defined in part by the contents of a mind-wandering episode, where the contents are unrelated to a task an agent was performing, or was supposed to perform. But there are various ways for an agent to engage in task-unrelated thought. Some categorize mind-wandering episodes in terms of a relationship to an agent’s intention: mind wandering might occur intentionally or unintentionally ( Giambra 1995 ; Seli et al. 2016 ). A second way to categorize mind-wandering episodes is in terms of a relationship to external stimuli. One might here distinguish between distraction, when the mind is prompted to wander by external stimuli, and mind wandering, when the mind is prompted to wander by internal processes, independently of any particular stimuli (see Stawarczyk et al. 2013 ). Or one could argue that distraction, especially sustained distraction, is a legitimate mind wandering as well.
A third way to characterize mind wandering is not in terms of its contents, but rather its dynamics. So, e.g., Christoff et al. (2016) characterize mind wandering as a species of spontaneous thought, with distinct dynamics. Mind wandering is distinguished from creative thought, and rumination, and other types of mental episodes, by relation to the presence or absence of various constraints on the episode (e.g., what they call “deliberate” and “automatic” constraints).
From a certain height, it appears that these different characterizations may not be in competition. Perhaps there are many routes to mind wandering. Perhaps some of them overlap. Perhaps different questions can be answered by focusing on certain routes in certain contexts. Reasonably, Seli et al. (2018) have recently argued in favor of mind wandering as a natural kind, with different sub-types grouped together by relations akin to family resemblance: “We propose that the field acknowledge mind-wandering to be a multidimensional and fuzzy construct encompassing a family of experiences with common and unique features” (2018, 482).
Methodological and conceptual clarity will simply require, in empirical manuscripts, something like the following sentence: “Here, we conceptualized mind-wandering as ________, and operationally defined it for our participants as ________.” Critically, this approach allows researchers the freedom to study whatever features of mind-wandering they wish, while providing the required specificity about aspects of the experience being explored. (488)
In the same spirit, I note here the sub-type of mind wandering that concerns me. I am interested in unintentional mind wandering—episodes of mind wandering that are neither initiated nor governed by any reportable intention of the agent. This category may cross-cut any relationship to external stimuli, in the sense that unintentional mind wandering could be externally or internally initiated. And it may demonstrate dynamics that are distinct from other sub-types of mind wandering.
Unintentional mind wandering could in principle happen non-consciously. But the literature on human mind wandering has it pegged as a feature of the conscious mind. That is to say, when the mind wanders, what wanders is the stream of consciousness—processes of conscious mentation. So, one key way to study mind wandering is to ask people whether or how often their mind has wandered. People offer reports about it. They recognize that they have been mind wandering. This is not because of mind wandering’s phenomenological signature. It is rather because people have a sense that they were once up to something, and then, more or less unbeknownst to them, they began to be up to something else. Thomas Metzinger (2013) speaks of this as the self-representational blink: an unnoticed shift from pursuing one task to doing whatever it is we do when the mind wanders. Recognizing that your mind has been wandering is always slightly surprising, because you did not plan for things to go in that way. From your perspective, it seems that they just did .
This is puzzling. But calling a mental episode unintentional need not imply that mind wandering is maladaptive, or that it has no function. Indeed, the very frequency with which it occurs had led many to suggest that it must have some functional role (e.g., Baird et al. 2011 ). It may not, of course. Perhaps, we survive in spite of how mentally addled we all are. But it is at least plausible that there is a function.
Some accounts of mind wandering might be taken to deny this. McVay and Kane (2010a ) and Kane and McVay (2012) , e.g., have argued that mind wandering reflects a failure of executive control. They note that a negative correlation exists between working memory capacity and a tendency to experience task-unrelated thoughts (see also Randall et al. 2014 ). Some such correlation is plausible. When one experiences task-unrelated mentation, something has clearly gone wrong. One has failed to stay on task.
But this also fails to imply that mind wandering has no function. Kane and McVay note that the correlation between working memory capacity and task-unrelated thought is not terribly strong: “WMC accounts for only about 5% of the variability in [task-unrelated thought] TUT rates (and vice versa)” (2012, 352). It is possible that mind wandering is both a failure in one sense and adaptive in another.
[W]e found evidence for the hypothesis that cognitive control abilities are specifically involved in the flexible adjustment of mind-wandering to task demands. As was hypothesized, high-WMC participants showed higher levels of TUT adjustment than did low-WMC participants. Thus, a more flexible coordination of the stream of thought appears to be characteristic of high-WMC individuals: They engage in TUTs when situational demands are low but reduce TUTs in attention-demanding situations. (1313)
This hypothesis is consistent with work that has demonstrated that as cognitive control resources diminish with age, the propensity to mind wander diminishes as well ( Maillet and Schacter 2016 ).
If we are to believe that mind wandering is associated with deployments of cognitive control, we need evidence that when agents mind wander, they engage in thought processes that may be beneficial. Some evidence for this is that when agents mind wander, their thoughts very frequently go to non-occurrent goals and needs, and to mentation about how to satisfy these goals in the future ( Klinger 1999 ; Baird et al. 2011 ).
Indeed, as Irving and Thompson (2019) note, it seems that it is possible to manipulate the content of mind wandering episodes by giving agents specific goals. Morsella et al. (2010) told some participants they would, in the near future, have to answer questions about the states in America. Then they gave the participants a different task. About 70 percent of these participants’ task-unrelated thoughts were about U.S. geography. Similarly, Mac Giolla et al. (2017) gave some participants a real future task, and told different participants to only pretend to have (or to lie about having) the same future task. Those participants with genuine intentions reported much more spontaneous thought about the future task than participants without genuine intentions.
It is also possible to manipulate mind wandering by reminding agents of their goals. Kopp et al. (2015) had participants either construct a list of their plans for the week or list features of a car. Participants then performed a reading task. Participants who had just reviewed a set of their own plans and goals reported much more mind wandering during the reading.
There is thus an apparent tension within mind wandering. When the mind wanders (at least unintentionally), agents are distracted from the current task, and performance suffers. But when the mind wanders, it tends to find non-occurrent goals the agent possesses, generating planning that could be beneficial. What’s more, greater cognitive control is associated with increases in mind wandering, especially when task demands are low.
Recall my original question: why does the mind wander? Two related questions that could help: What causes it to start, and what explains what happens as it wanders?
My proposed answer runs through recent work on cognitive control, and on what kinds of mechanisms drive allocations of cognitive control resources. I discuss this work in the next section.
A remarkable feature of the human cognitive system is its ability to configure itself for the performance of specific tasks through appropriate adjustments in perceptual selection, response biasing and the on-line maintenance of contextual information. The processes behind such adaptability, referred to collectively as cognitive control … ( Botvinick et al. 2001 , 624)
Rouault and Koechlin likewise emphasize processes of regulation towards certain ends: “Cognitive control refers to mental processes that evolve as regulating adaptive behavior beyond basic reinforcement and associative learning processes” (2018, 106).
There is a danger here, analogous to the one just discussed regarding definitions of mind wandering, in including far too many process-types under the same heading. “Cognitive control” includes processes like the construction and maintenance of a task set, the switching from one task set to another, the deployment of attention in various ways, the deployment of inhibition, and the monitoring of an agent’s progress towards goal achievement. To get better at understanding how these processes work together (or don’t), it helps to have a label. But the nature of the system is only loosely delineated.
Given this, there is room for differing emphases. So, e.g., Adele Diamond characterizes cognitive control processes as “a family of top-down mental processes needed when you have to concentrate and pay attention, when going on automatic or relying on instinct or intuition would be ill-advised, insufficient, or impossible” (136). This characterization is useful, but not definitive. For the kind of cognitive control processes, I have in mind here might be considered top-down, but do not activate only when agents need to deploy attention. These processes operate outside of the agent’s awareness, influencing the agent’s thought and action in subtle and difficult to detect ways.
So, e.g., Kurzban et al. (2013) have argued that one subtle way cognitive control mechanisms influence thought and action is by generating an experience of effort related to the performance of some task. They hypothesize that the experience of effort is the result of sub-personal computations that determine the current task’s value, as well as the value of nearby available tasks, and output a determination of the opportunity cost of persisting on the current task. The experience of effort is hypothesized to be a signal to the agent to switch tasks.
Kurzban et al. ’s proposal has received a lot of attention. Few agree with all of the specifics, but most agree with the general perspective that sub-personal monitoring mechanisms are concerned to determine the value of succeeding in the current task, as well as the cost of continuing engagement in the current task, and are concerned to, in some sense, direct the agent or her cognitive control resources in a more fruitful way.
Perhaps the most mature theory characterizing the mechanisms that constitute the allocation of cognitive control is the Expected Value of Control theory (see Shenhav et al. 2013 , 2017 ). The general idea is that the cognitive control system “specifies how much control to exert according to a rational cost-benefit analysis, weighing these effort costs against attendant rewards for achieving one’s goals” ( Lieder et al. 2018 , 2). Lieder et al. add to this idea a sophisticated model of how the cognitive control system might come to learn the value of the various control signals it can deploy, and might rely upon what it learns to guide cognition in adaptive ways.
Lieder et al. characterize the position the cognitive control system is typically in as a Markov decision process, specified over certain parameters, driven by reinforcement learning. Those parameters are the initial state of the system, the set of states the system could be in, the set of possible actions (or moves, or operations) the system could take, the conditional probabilities of transitioning between states, and a reward function. Lieder et al. further characterize the actions the system could take as “control signals that specify which computations the controlled systems should perform” (4).
Given this setup, the main aim is to maximize reward via the specification of control signals. The way the system does this is by way of learning algorithms. The system builds and updates a model that specifies transition probabilities between states given different control signals, and that maps these probabilities onto a reward function. The reward function balances the reward associated with an outcome (a new state), together with the computational costs of specifying the computation required to drive the system towards the outcome. So, what the system is designed to do is to take the action (specify the control signal or the package of control signals) that has the highest expected value, given the probabilities of where the action takes the system, and the costs of taking the action.
The hypothesis here is that “the cognitive control system learns to predict the context-dependent value of alternative control signals” (5), and that these predictions determine which actions the system takes.
In cases in which the context is relatively well-known, Lieder et al. posit that the system will depend upon relationships between features of the internal state of the agent and features of the context, and will perform weighted sum calculations to determine the value of various possible actions. Cases in which the context is not well-known are more difficult. But Lieder et al. propose that in such cases the system may utilize exploration strategies to teach itself the value of various actions in the novel situation. These exploration strategies involve drawing samples of the value of control signals in previously encountered contexts, averaging over them, and again selecting the control signal that provides the highest expected value.
Lieder et al. note that “This model is very general and can be applied to model cognitive control of many different processes” (6). And they offer a proof of concept for it, by demonstrating that their model outperforms alternative models across a range of processing types.
These processing types involve learning what features of a task are predictive of reward. Some of them are quite simple. One task on which their model performed well-involved learning where to allocate attention, based upon variable reward offered for attending to different locations. A second task involved learning the difference between colors that indicate reward, and colors that do not. That the model predicts basic learning of this sort is good, but not too surprising.
The expected value of computation depends not only on the rewards for correct performance but also on the difficulty of the task. In easy situations, such as the congruent trials of the Stroop task, the automatic response can be as accurate, faster, and less costly than the controlled response. In cases like this, the expected value of exerting control is less than the EVOC of exerting no control. By contrast, in more challenging situations, such as incongruent Stroop trials, the controlled process is more accurate and therefore has a positive EVOC as long as accurate performance is sufficiently important. Therefore, on incongruent trials the expected value of control is larger than the EVOC of exerting no control. Our model thus learns to exert control on incongruent trials but not on congruent trials. Our model achieves this by learning to predict the EVOC from features of the stimuli. This predicts that people should learn to exert more control when they encounter a stimulus feature (such as a color or word) that is predictive of incongruence than when they encounter a feature that is predictive of congruence. (19)
Of course, agents are rarely aware that a system (or coordinated collection of mechanisms) within them is busy learning the value of different modes of responding, and guiding the way that they deploy cognitive control resources. We are not here explaining explicit deliberation or planning. But we are getting insight into the processes—sub-personal, if you like—that create the cognitive ocean in which more explicit processes swim. What’s more, we are getting insight into the kinds of learning that drive cognitive control operations that agents have to simply live with. Shifts of attention, pulls to engage in various computational operations, a sense of what operations are valuable in what contexts—these are rarely things we explicitly consider. Rather, we depend upon this background to engage in explicit cognition and intentional action.
With this as background, I can suggest an interesting possibility, leading to a proposal regarding the etiology and function of mind wandering.
The possibility is this. Depending on the cognitive control system’s model of the value of various control signals, in cases containing relatively little expected value the system may select a package of control signals leading to exploration. These would be cases in which the goal is to find a new and better goal. And the method, which remains here unclear—although one could imagine it involving shifts of attention, construction of task sets involving imagination, inhibition of current goals, etc.—might be generally described as disengagement from the present task in order to set out upon a search for a more valuable task.
The cognitive control proposal, then, is this. Mind wandering is caused by the cognitive control system precisely when, and because, the expected value of whatever the agent is doing—usually, exercising control towards the achievement of some occurrent goal—is deemed too low, and this “too low” judgment generates a search for a better goal, or task. Perhaps, e.g., the estimation of expected value dips below a value threshold attached to the package of control signals that generate exploration for another goal, or task. Or perhaps the value is always computed in comparison with available options, such that mind wandering is sometimes initiated even in the face of a rewarding current task.
This is a straightforwardly empirical proposal, and should be assessed in terms of the explanations it affords, and by whether the predictions it makes are confirmed or disconfirmed. Before I discuss explanation and prediction, however, I wish to note two things.
First, it would certainly be useful if the cognitive control system contained such an operation. Humans are sophisticated agents, with multiple needs and goals potentially in play in most waking life situations. Fixation on one goal alone, or working towards the satisfaction of one goal at a time, is not a great strategy for flourishing. For, first, if one gets stuck on a difficult goal, or if it becomes apparent (i.e. apparent at least to some system tasked with calculating such a thing) that the present goal is not as rewarding as once calculated, it is much wiser to disengage and seek a better goal. And, second, in many situations progress towards multiple goals at once is possible. All one needs is the capacity to divide attention somewhat, or the capacity to hold multiple goals in mind—or at least within some accessible place—and one might waste much less time. Notice, further, that the above points may hold even if dividing the mind amongst multiple goals leads to performance decrements. Perfect performance is not always required. So long as mediocre performance allows one to satisfy goals and needs, accepting mediocre performance will be a good strategy.
Second, explicit cognitive control already does contain such an operation. Sometimes a task becomes too effortful, too uncomfortable, or too boring. Sometimes—after one has just awakened from a long nap, e.g.—there’s no obvious task at hand. In such cases performing a search for a high-value goal is a familiar operation that we perform explicitly. In other cases, we do not leave behind the current task, but we rather utilize deliberation, prospection, imagination, and other processes in order to find sub-goals, or means to achieve the goal that is currently structuring behavior. These modes of exploration towards discovery of a high-value goal are explicit. Our question here is whether the cognitive control system implicitly—i.e., in the absence of an explicit or conscious formation of intention to do so—initiates mind wandering as a similar mode of exploration, and for similar reasons. The proposal is that it does.
Here are explanations this proposal affords.
First, this proposal offers an explanation for the initiation of mind wandering episodes. These episodes are initiated without the agent’s explicit consent. But they do frequently occur. One possible explanation is that the agent necessarily loses control in these instances. That characterizes the initiation of a mind wandering episode as random. A better explanation, I submit, is that while the initiation of a mind wandering episode is, in one sense, a failure—a failure of the current goal and task set to persist—it is, in another sense, a smart move. It is smart because it results from a cognitive control system that is more or less constantly attempting to determine the value of selecting packages of control signals, and that will act when discrepancies in value are calculated. Note, incidentally, that this could be extended to cases in which the agent is pursuing no particular goal, or has no current task. The system need not always compare value between goals. It might be useful, e.g., to tag expected levels of reward to particular environments, perhaps by averaging over the kinds of rewards an environment-type provides. If agents associate one type of environment—a party, e.g.,—to a plethora of rewarding experiences, then a signal that this environment is near—one can hear party music, e.g.,—might lead the mind to wander in the direction of the kinds of experiences the rewarding environment provides.
The fact that the initiation of mind wandering episodes is smart helps to additionally explain a second fact, namely, that agents with higher levels of cognitive control mind wander more frequently when the current task is easy or non-rewarding.
This is not to deny that mind wandering episodes may sometimes be initiated by affectively salient stimuli, or other distractors. Nor is it to deny the existence of completely unguided, or otherwise guided, episodes of mind wandering. I am not in a position to deny that, e.g., a case of spreading activation in a semantic network could qualify as unintentional mind wandering. It may very well be—indeed it seems plausible—that only some cases of unintentional mind wandering are controlled in the way I here propose. Note, however, that even if this is right, the cognitive control system may be able to interact with uncontrolled mind wandering processes. In some cases, uncontrolled mind wandering could be commandeered if a valuable goal suggests itself.
Third, this proposal offers an explanation for the fact that mind wandering episodes tend to go to other goals the agent possesses. This is a natural place for a process to go if that process is structured by an aim to find a more rewarding goal than the one from which the agent has just disengaged. For it will be much more cost-effective to find existent goals, perhaps by querying memory, than to explore the environment and to construct entirely new goals (although of course this may happen, especially when the environment easily affords novel and rewarding goals).
Fourth, this proposal might be integrated with extant explanations of aspects of mind wandering. Consider, e.g., the decoupling hypothesis ( Antrobus et al. 1970 ; Smallwood et al. 2003 ; Smallwood and Schooler 2006 )—the idea that once mind wandering is underway, domain-general cognitive processes are engaged to maintain the mind wandering episode, by keeping attention decoupled from perceptual input, and by aiding the “continuity and integrity” of the agent’s train of thought ( Smallwood 2013 , 524). As Smallwood (2013) notes, the decoupling hypothesis does not seek to explain the initiation of mind wandering. The cognitive control proposal is consistent with it. That is, the proposal is consistent with domain-general resources being deployed to assist mind wandering episodes. The main comment I wish to make here is that the decoupling hypothesis becomes more plausible, and data on the deployment of domain-general resources in mind wandering more transparent, if the entire process of mind wandering can be seen as goal-directed, where the goal is set by the cognitive control system.
This proposal is also consistent with work on the recruitment of neural areas during mind wandering. Christoff et al. , e.g. ( Christoff et al. 2009 ; Fox et al. 2015 ), have found that episodes of mind wandering recruited not only core areas of the default mode network—medial PFC, posterior cingulate/precuneus, and posterior temporoparietal cortex—but also dorsal anterior cingulate cortex and dorsolateral prefrontal cortex, “the 2 main regions of the executive network” ( Christoff et al. 2009 , 8722). Christoff et al. plausibly link the involvement of the executive network with task performance decrements. The cognitive control proposal adds the possibility that executive network recruitment is associated with the goal-directed nature of (at least some) unintentional mind wandering.
Consider, further, recent work on the dynamics of mind wandering. In a recent review, Christoff et al. (2016) rightly notice that much research on mind wandering has been content-based, “assessing the contents of thoughts in terms of their relationship to an ongoing task or activity” (722). They seek, instead, to offer a taxonomy of thought-types in terms of their dynamics—of how they operate over time. They propose two dimensions along which the dynamics of thought may be influenced. The first dimension is characterized in terms of the degree to which thought is constrained by mechanisms that are “flexible, deliberate, and implemented through cognitive control” (719). The paradigm here is the intentional generation of a deliberative process, or the intentional maintenance of attention on a task. The second dimension is characterized in terms of the degree to which thought is constrained by mechanisms that are automatic, in that they “operate outside of cognitive control to hold attention on a restricted set of information” (719). There are many ways thought may be automatically distracted—Christoff et al. mention affectively salient stimuli as one example.
Within our framework, mind-wandering can be defined as a special case of spontaneous thought that tends to be more-deliberately constrained than dreaming, but less-deliberately constrained than creative thinking and goal-directed thought. In addition, mind-wandering can be clearly distinguished from rumination and other types of thought that are marked by a high degree of automatic constraints, such as obsessive thought. (719)
Now, this is not an explanation of why the mind wanders. It is, instead, a mapping of mind wandering onto a broader taxonomy of cognitive kinds, with special attention given to other modes of spontaneous thought. This taxonomy is useful for a number of reasons. For example, Christoff et al. map their taxonomy onto areas of the brain. So they say, e.g., that the part of the default network that centers on the medial temporal lobe is likely to be involved in the generation of mind wandering, as well as, via “its involvement in contextual associative processing” (724), the conceptual variability of some episodes of mind wandering. They also link the hippocampus to mind wandering, suggesting that it may contribute to the “imaginative construction” of hypothetical scenarios. Such mapping work from aspects of spontaneous thought onto activity patterns in large-scale brain networks affords fruitful suggestions for future study of the kinds of psychological patterns and activities that characterize mind wandering over time.
But there are possibilities and explanations that this approach does not (yet) address, and that potentially have consequences for the taxonomy of cognitive kinds that they offer.
Creative thinking may be unique among other spontaneous-thought processes because it may involve dynamic shifts between the two ends of the spectrum of constraints. The creative process tends to alternate between the generation of new ideas, which would be highly spontaneous, and the critical evaluation of these ideas, which could be as constrained as goal-directed thought in terms of deliberate constraints and is likely to be associated with a higher degree of automatic constraints than goal-directed thought because creative individuals frequently use their emotional and visceral reactions (colloquially often referred to as “gut” reactions) while evaluating their own creative ideas. (Box 1, 720)
I suggest that mind wandering is similarly complex. If the cognitive control proposal is correct, then in at least some cases mind wandering is initiated by processes of cognitive control, even though the goal driving mind wandering is not set explicitly by the agent. This could be captured by adding layers onto Christoff et al. ’s taxonomy, deepening explanations of the etiology and function of each kind of spontaneous thought. And these deeper explanations at each place could be expected to bear fruit for understanding the dynamics of spontaneous thought. In particular, we might hope to find patterns in the neural dynamics that are predictive of the onset as well as the termination of mind wandering episodes, and that differentiate it from dreaming, creative thought, and perhaps from rumination. If the cognitive control proposal is correct, one task would be to map these patterns onto the expected value calculations the cognitive control system is performing. We would expect the dynamics of mind wandering to reflect the initiation of a search for a more rewarding goal, and to reflect attempts to make progress on this search. But now I’m jumping ahead, to predictions the proposal generates.
The cognitive control proposal makes predictions. Confirmation of these would be good news; disconfirmation would be bad news.
First, given the explanation offered for the initiation of mind wandering episodes, the proposal predicts that increases in reward for satisfying an occurrent goal would correlate with decreases in propensity to mind wander. It is well-confirmed that increasing reward leads to boosts in performance level, and to overcoming any purported “ego-depletion,” even for very boring tasks. Paradigms that have established this result could be used to test for the place of mind wandering in the behavioral data.
Second, the proposal predicts that increases in reward for non-occurrent goals the agent possesses would increase mind wandering. We have already seen that reminding agents of goals they possess, or of goals they will soon need to attempt to satisfy, leads to more mind wandering in the direction of these goals. The prediction here is more specific. If one were to, e.g., notify participants that they were soon to perform a task associated with some level of reward, and then to put participants through a low reward task, the prediction is that tendency to mind wander towards this task would be associated with the discrepancy in reward between the current and upcoming task.
Third, this proposal draws upon a view of the cognitive control system on which the learning of values associated with goals, and the learning of values associated with stimuli features predictive of goals, is crucial. So the proposal, plus plausible assumptions about reinforcement learning processes, predicts that it is possible to train participants to associate stimuli with certain goals, and that registration of such stimuli would generate mind wandering to the degree that the associated goal is rewarding. Very costly goals would produce little mind wandering. Cheap but rewarding goals would produce more.
And it may be possible to extend this result. It depends on what the agent associates with rewarding goals. Above I suggested that the system need not always compare value between explicit goals, and that the value computation might include an association between expected levels of reward and particular environments. If so, simply placing an agent in such environments would manipulate levels of unintentional mind wandering.
It may be useful to distinguish predictions this proposal makes from a related proposal: the current concerns hypothesis. The current concerns hypothesis (for which, see Klinger et al. 1973 ; Smallwood and Schooler 2006 ) has it that mind wandering is caused by a shift in salience—when one’s current goals (or concerns: here I use these terms interchangeably), become more salient than the external environment, one’s mind begins to wander. As Smallwood explains the view, “attention will be most likely to shift to self-generated material when such information offers larger incentive value than does the information in the external environment” (2013, 524). This proposal is distinct from mine in the following ways. First, I propose a specific mechanism, connected with recent modeling work in cognitive control, to explain the onset of mind wandering. Thus far, of course, the proposal can be seen as a specification of the current concerns hypothesis. Second, this mechanism initiates mind wandering not by turning attention to one’s current concerns, but by directed thought to search for a more valuable goal than the present one. So the cognitive control proposal makes predictions the current concerns hypothesis does not. For example, the cognitive control proposal predicts that propensity to mind wander could be increased by devaluing the present goal, independently of the salience of any of one’s current goals. That is, no matter how much one’s current goals or concerns lack salience, once could increase mind wandering by devaluing the occurrent goal. And it predicts that mind wandering will not turn directly to one’s other goals—the mind may wander to the environment, rather than to internal concerns, since this is one way the agent may attempt to find a more rewarding task. So we should, e.g., be able to find episodes of more intense environmental scanning as a part of the mind wandering episode. Indeed, if the environment is expected to contain valuable options, one would predict that this is where attention will go, rather than to any internal space of concerns.
This is not to deny that mind wandering represents a failure in some sense. McVay and Kane (2010b ) have argued that mind wandering represents an executive control failure. What fails is a process of goal maintenance: “we suggest that goal maintenance is often hijacked by task-unrelated thought (TUT), resulting in both the subjective experience of mind wandering and habit-based errors” (324). The possibility I am raising is that failures of goal-maintenance could in another sense be successes of a different process. Indeed, perhaps processes of goal-maintenance are closely related to the value-based process of estimating the expected value of continuing on some task, or of searching for a new task, that I propose underlies unintentional mind wandering.
In sum, the proposal is plausible on its face. If correct, it promises to explain a range of data regarding mind wandering, and to explain the—from the agent’s conscious perspective very puzzling—initiation of mind wandering episodes. The proposal may also contribute to explanations of the dynamics of mind wandering. The predictions this proposal makes are testable, and work in this direction might take steps towards further integrating knowledge of how cognitive control works with knowledge of how mind wandering works.
I wish finally to relate this proposal to two leading philosophical accounts of mind wandering. Both of these accounts aim to capture mind wandering quite generally. I have noted in Mind wandering section that this is not my aim. Here, I want only to discuss implications for these more general accounts of mind wandering, if the cognitive control proposal about unintentional mind wandering is on track.
[T]he ability to control the conscious contents of one’s mind in a goal-directed way, by means of attentional or cognitive agency. This ability can be a form of rational self-control, which is based on reasons, beliefs, and conceptual thought, but it does not have to be. What is crucial is the “veto component”: Being mentally autonomous means that all currently ongoing processes can in principle be suspended or terminated. This does not mean that they actually are terminated, it just means that the ability, the functional potential, is given and that the person has knowledge of this fact. M-autonomy is the capacity for causal self-determination on the mental level. (2013, 4)
I think the brush strokes Metzinger uses are too broad. I doubt we have veto control over every conscious process ongoing at a time. But I do think he locates an interesting phenomenon. In unintentional mind wandering, our knowledge (or awareness) that we might suspend, terminate, or re-direct aspects of the stream of consciousness lapses.
My question is this. Should we think of this lapse as the agent’s loss of control? As Metzinger has it, mind wandering essentially involves a lack of ability, and a lack of control—what he calls veto control. I agree that unintentional mind wandering does involve a loss of one kind of control. But I would underline the fact that there are multiple ways for a system to exercise control. Some of these involve consciousness in crucial ways. Some likely do not ( Shepherd 2015 ). Knowledge that one can exercise control in some way at a moment can be useful. But a system may be well-designed, and exercise control in finding or executing goals, even if the system is not explicitly aware of processes that are performing these functions at a time.
Further, there are multiple ways for a system or an agent to possess an ability. The mind wandering agent may lack the ability to suspend, terminate, or re-direct elements of the stream of consciousness in virtue of her knowledge or awareness that she can do so. But she may retain the ability to suspend, terminate, or re-direct elements of the stream of consciousness in virtue of other features—perhaps in virtue of signals that emanate from the cognitive control processes I have emphasized.
This is not a merely verbal distinction. It is about how we understand the constitution of agency, and the kinds of properties that should be ascribed to mind wandering. If the cognitive control proposal is right, mind wandering emerges as an interesting case in which the seams of agency pull apart somewhat—we fail to notice that a non-conscious mechanism has turned the stream of consciousness in a different direction. But there may be good functional reasons for this operation, and it may contribute to an agent’s overall capacities to control the self in various environments and contexts.
An agent A’s attention is unguided if and only if A is not habitually guided to focus her attention on any information. In particular, she does not satisfy the counter-factual condition for attentional guidance: There is no information i such that, if A’s attention isn’t focused on i, she will notice, feel discomfited by, and thereby be disposed to correct this fact. (567)
I am not sure this is right. Mind wandering episodes are sometimes short. Sometimes they stop, it seems to me, precisely because we feel a sense that we were recently up to something, and we feel a pull to return. The cognitive control proposal might be able to explain this—one good move for the cognitive control system, in case of a failure to find a more rewarding task or goal, would be to return to the previous task.
Irving is aware that when it wanders, the mind frequently circles back to the agent’s goals. Does this not suggest guidance of some sort? Irving explains the tension by distinguishing between guidance and motivation. Motivated behavior only requires that an agent’s beliefs, desires, or goals are causal antecedents of the behavior. Guided behavior, by contrast, is explicated in terms of dynamics: it “involves the online monitoring and regulation of behavior” (563). Irving claims that mind wandering may be motivated, but it is not guided.
This aspect of Irving’s account does not compare favorably with the cognitive control proposal—if, of course, future work confirms the proposal. For Irving’s account offers no explanation of how causation by some belief or desire or goal helps explain how or why the wandering mind frequently turns to the agent’s goals. The cognitive control proposal has it that the wandering mind finds goals because that aim is what initiated and governs the mind wandering episode.
Further, if my proposal is right it is not entirely correct to think of mind wandering as unguided. It is, admittedly, not guided by any explicit intention the agent forms. In one sense of “guided,” then, Irving is right. But on the cognitive control proposal, mind wandering is a cognitive control process, and it does have a purpose. It seems purposeless to us in part because it is an interesting case in which some of the seams of agency pull apart somewhat—we do not notice that a non-conscious mechanism has turned the stream of consciousness in a different direction. And it seems purposeless to us in part because the course of the stream of consciousness during mind wandering is, as the cognitive control system plans it, meandering. It is meandering because the goal is to search, to explore, until a more rewarding task is found.
If these considerations are on track, we should say that mind wandering takes the form of a conscious but non-consciously guided process the aim of which is to find a rewarding goal or task. The connection with the cognitive control system explains the guidance aspect—the functionality of mind wandering—and affords the possibility of integration with work on the dynamics of mind wandering. The non-conscious aspect of the guidance explains the air of mystery surrounding mind wandering, why it seems purposeless, and why it seems to come about randomly.
In this article, I have asked why the mind wanders. I focused on a sub-type of mind wandering—mind wandering that occurs independently of any reportable intention. I proposed that unintentional mind wandering is sometimes initiated and sustained by aspects of cognitive control. Unintentional mind wandering is caused by the cognitive control system precisely when, and because, the expected value of whatever the agent is doing—usually, exercising control towards achievement of some occurrent goal—is deemed too low, and this “too low” judgment generates a search for a better goal, or task.
This proposal generates testable predictions, and suggests open possibilities regarding the kinds of computations that may underlie unintentional mind wandering. My hope is that by connecting research on mind wandering with research on cognitive control resource allocation, fruitful strategies for modeling these computations may be taken from cognitive control research and deployed to help explain the initiation and dynamics of mind wandering episodes.
The cognitive control proposal also points us towards a fuller picture of human agency. In this picture, action control and intelligent thought are stitched together by conscious and non-conscious processes operating in concert. Future empirical work is critical to the confirmation of this picture, and to filling in the many unspecified details. This is so not least because, if the proposal I offer is on track, agents are not introspectively aware of the (good) rationale behind many mind-wandering episodes.
The author acknowledges two sources of support. First, funds from European Research Council Starting Grant 757698, awarded under the Horizon 2020 Programme for Research and Innovation. Second, the Canadian Institute for Advanced Research’s Azrieli Global Scholar programme on Mind, Brain, and Consciousness.
Conflict of interest statement . None declared.
Antrobus JS , Singer JL , Goldstein S , et al. Mindwandering and cognitive structure . Trans N Y Acad Sci 1970 ; 32 : 242 – 52 .
Google Scholar
Baird B , Smallwood J , Schooler JW. Back to the future: autobiographical planning and the functionality of mind-wandering . Conscious Cogn 2011 ; 20 : 1604 – 11 .
Boyd R. Realism, anti-foundationalism and the enthusiasm for natural kinds . Philos Stud 1991 ; 61 : 127 – 48 .
Botvinick MM , Braver TS , Barch DM , et al. Conflict monitoring and cognitive control . Psychol Rev 2001 ; 108 : 624.
Braem S , Verguts T , Roggeman C , et al. Reward modulates adaptations to conflict . Cognition 2012 ; 125 : 324 – 32 .
Christoff K , Gordon AM , Smallwood J , et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering . Proc. Natl. Acad. Sci 2009 ; 106 : 8719 – 24 .
Christoff K , Irving ZC , Fox KC , et al. Mind-wandering as spontaneous thought: a dynamic framework . Nat Rev Neurosci 2016 ; 17 : 718.
Christoff K , Mills C , Andrews-Hanna JR , et al. Mind-wandering as a scientific concept: cutting through the definitional haze . Trends Cogn Sci 2018 ; 22 : 957 – 9 .
Fox KC , Spreng RN , Ellamil M , et al. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes . Neuroimage 2015 ; 111 : 611 – 21 .
Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought . Conscious Cogn 1995 ; 4 : 1 – 21 .
Irving ZC. Mind-wandering is unguided attention: accounting for the “purposeful” wanderer . Philos Stud 2016 ; 173 : 547 – 71 .
Irving ZC , Thompson E ( 2019 ). The philosophy of mind wandering. In: Kieran F , Kalina C (eds), Oxford Handbook of Spontaneous Thought and Creativity . New York, NY : Oxford University Press .
Google Preview
Killingsworth MA , Gilbert DT. A wandering mind is an unhappy mind . Science 2010 ; 330 : 932.
Kane MJ , McVay JC. What mind wandering reveals about executive-control abilities and failures . Curr Direct Psychol Sci 2012 ; 21 : 348 – 354 .
Klinger EC. Thought flow: properties and mechanisms underlying shifts in content. In: Singer JA , Salovey P (eds), At Play in the Fields of Consciousness: Essays in the Honour of Jerome L. Singer . Mahwah, NJ : Erlbaum , 1999 , 29 – 50 .
Klinger E , Gregoire KC , Barta SG. Physiological correlates of mental activity: Eye movements, alpha, and heart rate during imagining, suppression, concentration, search, and choice . Psychophysiology 1973 ; 10 : 471 – 477 .
Kopp K , D’Mello S , Mills C. Influencing the occurrence of mind wandering while reading . Conscious Cogn 2015 ; 34 : 52 – 62 .
Kurzban R , Duckworth A , Kable JW , et al. An opportunity cost model of subjective effort and task performance . Behav Brain Sci 2013 ; 36 : 661 – 679 .
Levinson DB , Smallwood J , Davidson RJ. The persistence of thought: evidence for a role of working memory in the maintenance of task-unrelated thinking . Psychol Sci 2012 ; 23 : 375 – 380 .
Lieder F , Shenhav A , Musslick S , et al. Rational metareasoning and the plasticity of cognitive control . PLoS Comput Biol 2018 ; 14 : e1006043.
Mac Giolla E , Granhag PA , Ask K. Task-related spontaneous thought: a novel direction in the study of true and false intentions . J Appl Res Mem Cogn 2017 ; 6 : 93 – 103 .
Maillet D , Schacter DL. From mind wandering to involuntary retrieval: age-related differences in spontaneous cognitive processes . Neuropsychologia 2016 ; 80 : 142 – 156 .
Meier ME. Is there a positive association between working memory capacity and mind wandering in a low-demand breathing task? A preregistered replication of a study by Levinson, Smallwood, and Davidson (2012) . Psychol Sci 2019 ; 30 : 789 – 797 .
McVay JC , Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) . Psychol Bull 2010a ; 136 : 188 – 197 .
McVay JC , Kane MJ. Adrift in the stream of thought: the effects of mind wandering on executive control and working memory capacity. In: Gruszka A, Matthews G, Szymura B (eds), Handbook of Individual Differences in Cognition. New York, NY : Springer , 2010b , 321 – 34 .
Metzinger TK. The myth of cognitive agency: subpersonal thinking as a cyclically recurring loss of mental autonomy . Front Psychol 2013 ; 4 : 931.
Morsella E , Ben-Zeev A , Lanska M , et al. The spontaneous thoughts of the night: how future tasks breed intrusive cognitions . Soc Cogn 2010 ; 28 : 641 – 650 .
Murray S , Krasich K , Schooler JW , et al. Conceptual and methodological considerations for investigations of the task-unrelated-thought (TUT) variety of mind wandering, 2019 ; doi: 10.31234/osf.io/fsg57.
Randall JG , Oswald FL , Beier ME. Mind-wandering, cognition, and performance: a theory-driven meta-analysis of attention regulation . Psychol Bull 2014 ; 140 : 1411.
Rouault M , Koechlin E. Prefrontal function and cognitive control: from action to language . Curr Opin Behav Sci 2018 ; 21 : 106 – 111 .
Rummel J , Boywitt CD. Controlling the stream of thought: Working memory capacity predicts adjustment of mind-wandering to situational demands . Psychon Bull Rev 2014 ; 21 : 1309 – 1315 .
Seli P , Risko EF , Smilek D , et al. Mind-wandering with and without intention . Trends Cogn Sci 2016 ; 20 : 605 – 617 .
Seli P , Beaty RE , Cheyne JA , et al. How pervasive is mind wandering, really? Conscious Cogn 2018 ; 66 : 74 – 78 .
Seli P , Carriere JS , Wammes JD , et al. On the clock: evidence for the rapid and strategic modulation of mind wandering . Psychol Sci 2018 ; 29 : 1247 – 1256 .
Shenhav A , Botvinick MM , Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function . Neuron 2013 ; 79 : 217 – 240 .
Shenhav A , Musslick S , Lieder F , et al. Toward a rational and mechanistic account of mental effort . Annu Rev Neurosci 2017 ; 40 : 99 – 124 .
Shepherd J. Conscious control over action . Mind Lang 2015 ; 30 : 320 – 344 .
Smallwood J. Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity . Psychol Bull 2013 ; 139 : 519 – 535 .
Smallwood J , Baracaia SF , Lowe M , et al. Task unrelated thought whilst encoding information . Conscious Cogn 2003 ; 12 : 452 – 484 .
Smallwood J , Schooler JW. The restless mind . Psychol Bull 2006 ; 132 : 946 – 958 .
Stawarczyk D , Cassol H , D'Argembeau A. Phenomenology of future-oriented mind-wandering episodes . Front Psychol 2013 ; 4 : 425.
Email alerts
Citing articles via.
- About Association for the Scientific Study of Consciousness
Affiliations
- Online ISSN 2057-2107
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
Abstract. Mind wandering and mindfulness are often described as divergent mental states with opposing effects on cognitive performance and mental health. Spontaneous mind wandering is typically associated with self-reflective states that contribute to negative processing of the past, worrying/fantasizing about the future, and disruption of ...
The Intentionality of Mind-Wandering. Research on mind-wandering has seen a massive increase in recent years, spreading to a wide variety of psychological domains including those examining cognition [1-10], neuroscience [11-16], education [17-20], creativity [21,22], clinical populations [23-26], and workplace functioning [], to name a few.. The rapidly growing body of research on mind ...
Most research on mind-wandering has characterized it as a mental state with contents that are task unrelated or stimulus independent. However, the dynamics of mind-wandering — how mental states ...
Research also suggests that mind-wandering serves as a mnemonic process, involving a variety of episodic forms of autobiographical memory facilitating an «autonoetic consciousness» unique to the human capacity of the awareness of self (Tulving & Craik, 2005; Vago & Zeidan, 2016).
Mind wandering is a universal phenomenon in which our attention shifts away from the task at hand toward task-unrelated thoughts. Despite it inherently involving a shift in mental set, little is known about the role of cognitive flexibility in mind wandering. In this article we consider the potential of cognitive flexibility as a mechanism for mediating and/or regulating the occurrence of mind ...
Conscious experience is fluid; it rarely remains on one topic for an extended period without deviation. Its dynamic nature is illustrated by the experience of mind wandering, in which attention switches from a current task to unrelated thoughts and feelings. Studies exploring the phenomenology of mind wandering highlight the importance of its content and relation to meta-cognition in ...
Two such forms of spontaneous thought include involuntary autobiographical memories (IAMs; Berntsen, 1996, 2010) and mind wandering (MW; Smallwood & Schooler, 2006).IAMs have been defined as memories of one's personal past that are retrieved unintentionally (i.e., "without preceding attempts at retrieving it"; Berntsen, 1996, p. 435).In a similar fashion, MW has been defined by "a ...
Mind-wandering (MW) as a research topic has received considerable attention over the last several decades. The recent differentiation between spontaneous and deliberate MW has suggested a particular effect of the former on psychopathology; in that increased spontaneous MW may precede mental illness. The present study sought to explore MW as a ...
Mind-wandering—defined as off-task thinking—can be disruptive to daily functioning. Mindfulness is considered a potential method for reducing mind-wandering; however, no study has systematically reviewed findings on this topic. The present systematic review synthesizes current findings from this literature, examining whether results vary as a function of study methodology. Our final sample ...
Q&A — Psychologist Jonathan Smallwood. The science of a wandering mind. More than just a distraction, mind-wandering (and its cousin, daydreaming) may help us prepare for the future. When psychologist Jonathan Smallwood set out to study mind-wandering about 25 years ago, few of his peers thought that was a very good idea.
Mind Wandering and Other Lapses. J. Smallwood, in Encyclopedia of Consciousness, 2009 Mind wandering is a universal human experience in which the focus of attention temporarily shifts from what we are doing. This article describes how to conceptualize these shifts in attention as changes in the flow of information through an attentional system and considers the different explanations offered ...
In many situations, increasing task difficulty decreases thoughts that are unrelated to the task (i.e., mind-wandering). In the context of reading, however, recent research demonstrated that increasing passage reading difficulty actually increases mind-wandering rates (e.g., Feng et al. in Psychon Bull Rev 20:586-592, 2013). The primary goal of this research was to elucidate the mechanism ...
New research led by UC Berkeley has come up with a way to track the flow of our internal thought processes and signal whether our minds are focused, fixated or wandering. Using an electroencephalogram (EEG) to measure brain activity while people performed mundane attention tasks, researchers identified brain signals that reveal when the mind is ...
A scientist says mind-wandering or daydreaming help prepare us for the future. Tim Vernimmen, Knowable Magazine. Scientists are beginning to understand when and why minds start to wander. Knowable ...
Mind wandering (MW) and mindfulness have both been reported to be vital moderators of psychological wellbeing. Here, we aim to examine how closely associated these phenomena are and evaluate the ...
For mind wandering, researchers have distinguished between intentional and unintentional aspects of mind wandering (Seli, Carriere, & Smilek, 2015), although this distinction has not been considered in the context of nature. Thus, little is known about which facets of mindfulness and mind wandering strengthen the outcomes of nature experiences.
tion: mind-wandering research came into prominence within both mainstream psychology20,21 and cognitive neuroscience22,23. However, this new research inherited
Mind-wandering is important in understanding how the brain produces what William James called the train of thought and the stream of consciousness. This aspect of mind-wandering research is focused on understanding how the brain generates the spontaneous and relatively unconstrained thoughts that are experienced when the mind wanders.
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. Nevertheless, the scientific exploration of MW poses unique challenges; MW is by nature a spontaneous, off task, internal mental process which is often unaware and usually difficult to ...
Mind wandering minimizes mind numbing: Reducing semantic-satiation effects through absorptive lapses of attention. Mind-wandering and meta-awareness in hypnosis and meditation: Relating executive function across states of consciousness. Mind wandering "Ahas" versus mindful reasoning: alternative routes to creative solutions.
The authors suggest that mind wandering might draw attention away from sensory input toward task-unrelated mental representations, impairing perception. While this may be problematic during tasks that require precise timing with respect to the environment, like driving, perceiving time intervals as shorter could be beneficial when trying to ...
The current study aimed to identify the effectiveness of attention training (training on focusing and switching attention) in reducing mind wandering during e-learning among university students. The research sample consisted of (52) male and female university students who were randomly distributed equally among the experimental and control groups.
Even then, much of the initial research focused on the poorer end of the spectrum - people with aphantasia, who claim to lack a mind's eye. Within the past five years, however, interest in ...
Mind wandering is a dominant activity of our mental life that is present in a multiplicity of psychological conditions. The initial scientific studies of daydreaming proposed that people are in a state of mind wandering during a significant part of their waking life (Singer, 1974, 1975).Recent estimates found that people report that they are in a mind wandering state at roughly one third of ...
By Anna Kelsey-Sugg, Sana Qadar and Rose Kerr for All in the Mind Posted 4h ago 4 hours ago Mon 22 Apr 2024 at 10:00pm Susannah Breslin didn't know it, but while she played preschool games she was ...
Much has been written about common leadership styles and how to identify the right style for you, whether it's transactional or transformational, bureaucratic or laissez-faire. But according to ...
But there are some important considerations for readers to keep in mind whenever they come across a news story or research about generations: Generational categories are not scientifically defined. The boundaries that place one person in Gen Z and another in the Millennial generation are not precise, definitive or universally agreed on.
Consider, further, recent work on the dynamics of mind wandering. In a recent review, Christoff et al. rightly notice that much research on mind wandering has been content-based, "assessing the contents of thoughts in terms of their relationship to an ongoing task or activity" (722). They seek, instead, to offer a taxonomy of thought-types ...