
Medicare Provider Enrollment – Site Verification
by nCred | Medicare Provider Enrollment | 0 comments
Medicare Provider Enrollment News
CMS contracts with a third party to provide site visit services as an integral part of the Medicare Provider Enrollment process. The National Sive Visit Contractor (NSVC) will conduct site visits for all providers and suppliers except for Durable Medical Equipment (DMEPOS) which will continue to be inspected by the National Supplier Clearinghouse. MSM Security Services, LLC has the national site visit contract. MSM, or one of its subcontractors, will conduct a site verification and screening process according to Medicare guidelines to prevent questionable providers and suppliers from enrolling in the Medicare program. When an inspector shows up, he or she will have valid ID and a letter of authorization to begin the inspection. You may not copy or retain the ID or letter of authorization. You may contact MSM at any point if you have questions at 855-220-1074.
The site verification may be as quick as verifying your business location, or an inspector may physically show up to tour your clinic. The process ensures that providers aren’t able to Enroll as participating Medicare providers without an appropriate service location.
You may see full details in section 10.6.20 of the Medicare Program Integrity Manual .
Call nCred today at (423) 443-4525 to discuss your Medicare Provider Enrollment needs. We work with all specialties and have extensive experience processing Medicare applications.
From the Medicare Program Integrity Manual:
10.6.20 – Screening: On-site Inspections and Site Verifications (Rev. 11949; Issued: 04-13-23; Effective: 04-21-23; Implementation: 06-19-23)
The contractor shall review section 10.3 of this chapter for special instructions regarding site visits. In the event of a conflict, those instructions take precedence over those in this section 10.6.20.
A. DMEPOS Suppliers and IDTFs
The scope of site visits of DMEPOS suppliers and IDTFs shall continue to be conducted in accordance with existing CMS instructions and guidance. (For purposes of this section 10.6.20, the term “contractor” refers to the Medicare Administrative Contractor; the term “SVC” refers to the site visit contractor.)
B. Provider and Supplier Types Other Than DMEPOS Suppliers and IDTFs
For provider/supplier types other than DMEPOS suppliers and IDTFs – that must undergo a site visit pursuant to this section 10.6.20 and § 424.518, the SVC will perform such visits consistent with the procedures in this section 10.6.20. This includes all of the following:
(1) Documenting the date and time of the visit, and including the name of the individual attempting the visit.
(2) Photographing the provider/supplier’s business for inclusion in the provider/supplier’s file. All photographs will be date/time stamped.
(3) Fully documenting observations made at the facility, which could include facts such as (a) the facility was vacant and free of all furniture, (b) a notice of eviction or similar documentation is posted at the facility, and (c) the space is now occupied by another company.
(4) Writing a report of the findings regarding each site verification.
(5) Including a signed site visit report stating the facts and verifying the completion of the site verification.
In terms of the extent of the visit, the SVC will determine whether the following criteria are met: (i) the facility is open; (ii) personnel are at the facility; (iii) customers are at the facility (if applicable to that provider or supplier type); and (iv) the facility appears to be operational. This will require the site visitor(s) to enter the provider/supplier’s practice location/site rather than simply conducting an external review. If any of the four elements ((i) through (iv)) listed above are not met, the contractor will, as applicable – and using the procedures outlined in this chapter and in existing CMS instructions – deny the provider’s enrollment application pursuant to § 424.530(a)(5)(i) or (ii) or revoke the provider’s Medicare billing privileges under § 424.535(a)(5)(i) or (ii).
C. Operational Status
When conducting a site verification to determine whether a practice location is operational, the SVC shall make every effort to limit its verification to an external review of the location. If the SVC cannot determine whether the location is operational based on this external review, it shall conduct an unobtrusive site verification by limiting its encounter with provider or supplier personnel or medical patients.
The contractor must review and evaluate the site visit results received from the SVC prior to making a final determination. If it is determined (during the review and evaluation process) that the location is non-operational based on the site visit results but there is reason to proceed with the enrollment, the contractor shall provide the appropriate justification in the comment section of the Validation Checklist in PECOS. (For example, a second site visit determined the location to be operational; the provider only renders services in patient’s homes; etc.).
If the contractor is unsure of how to proceed based on its evaluation of the site visit results, it shall contact its PEOG BFL and copy its contracting officer’s representative (COR).
Site verifications should be done Monday through Friday (excluding holidays) during their posted business hours. If there are no hours posted, the site verification should occur between 9 a.m. and 5 p.m. If, during the first attempt, there are obvious signs that the facility is no longer operational, no second attempt is required. If, on the first attempt, the facility is closed but there are no obvious indications that the facility is non-operational, a second attempt on a different day during the posted hours of operation should be made.
E. Documentation
As indicated previously, when conducting site verifications to determine whether a practice location is operational, the SVC shall:
(i) Document the date and time of the attempted visit and include the name of the individual attempting the visit.
(ii) As appropriate, photograph the provider/supplier’s business for inclusion in the provider/supplier’s file on an as-needed basis. All photographs should be date/time stamped.
(iii) Fully document all observations made at the facility (e.g., the facility was vacant and free of all furniture, a notice of eviction or similar documentation was posted at the facility, the space is now occupied by another company, etc.).
(iv) Write a report of its findings regarding each site verification.
F. Determination
(In the event an instruction in this subsection F is inconsistent with guidance in section 10.6.6, 10.4.7 et seq., or 10.4.8, the latter three sections of instructions shall take precedence.)
If a provider/supplier is determined not to be operational or in compliance with the regulatory requirements for its provider/supplier type, the contractor shall revoke the provider/supplier’s Medicare billing privileges – unless the provider/supplier has submitted a change of information request that notified the contractor of a change in practice location. Within 7 calendar days of CMS or the contractor determining that the provider/supplier is not operational, the contractor shall update PECOS or the applicable claims processing system (if the provider/supplier does not have an enrollment record in PECOS) to revoke Medicare billing privileges and issue a revocation notice to the provider/supplier. The contractor shall afford the provider/supplier applicable appeal rights in the revocation notification letter.
For non-operational status revocations , the contractor shall use either 42 CFR § 424.535(a)(5)(i) or 42 CFR § 424.535(a)(5)(ii) as the legal basis for revocation. Consistent with 42 CFR § 424.535(g), the date of revocation is the date on which CMS or the contractor determines that the provider/supplier is no longer operational. The contractor shall establish a 2-year reenrollment bar for providers/suppliers that are not operational.
For regulatory non-compliance revocations , the contractor shall use 42 CFR § 424.535(a)(1) as the legal basis for revocation. Consistent with 42 CFR § 424.535(g), the date of revocation is the date on which CMS or the contractor determines that the provider/supplier is no longer in compliance with regulatory provisions for its provider/supplier type. The contractor shall establish a 2-year enrollment bar for providers/suppliers that are not in compliance with provisions for their provider/supplier type.
G. Multiple Site Visits
Notwithstanding any other instruction to the contrary in this chapter, the contractor shall not order a site visit if the specific location to be visited has already undergone a successful site visit within the last 12 months and the applicable provider/supplier is in an approved status.
Consider the following illustrations:
Example 1 – A single-site home health agency (HHA) undergoes a revalidation site visit on February 1. The HHA submits a change of information request on July 1 to add a branch location. The contractor shall order this site visit because the visit will occur at a location (i.e., the branch location) different from the main location (i.e., the location that underwent the February 1 revalidation visit).
Example 2 – A DMEPOS supplier undergoes a revalidation site visit on April 1. It submits an initial Form CMS-855S application on May 1 to enroll a second location. The new location shall undergo a site visit because: (1) it is different from the first (revalidated) location; and (2) it is/will be separately enrolled from the first location.
Example 3 – A physical therapy (PT) group has three locations – X, Y, and Z. As part of a revalidation, the contractor elects to order a site visit of Location Y rather than X or Z. The visit was performed on June 1. On October 4, the group submits a Form CMS-855B to report a change of ownership, thus requiring a site visit under this chapter. However, the contractor shall not order a visit for Location Y because this site has been visited within the past 12 months. Location X or Location Z must instead be visited.
Example 4 – An IDTF undergoes an initial enrollment site visit on July 1. On September 24, it submits a Form CMS-855B application to change its practice location; this mandates a site visit under this chapter. The site visit shall be performed even though the initial visit took place within the past 12 months. This is because the second visit will be of the new location, whereas the first visit was of the old location.
H. Certified Providers/Suppliers – Address Validation Error
Notwithstanding any other instruction to the contrary in this chapter, the contractor need not order a site visit for a certified provider/supplier prior to making a recommendation to the state if an address validation error is received in PECOS. The contractor shall override the error message and notate in the referral package that the address was unverifiable. This avoids multiple site visits being performed (that is, pre-enrollment, survey, and post enrollment).
Share this:
Recent Posts
- Change Healthcare Network Disruption
- MHC and MFT Medicare Enrollment Standards
- Medicare Provider Enrollment Changes for 2024
- AZ Medicaid Extends Enrollment Moratorium for Behavioral Health
- Massachusetts Removes Invasive Credentialing Questions for Healthcare Practitioners
Credentialing Resources
- CAQH – UPD Provider Login
- Centers for Medicare and Medicaid Services
- Medicare Provider Enrollment Center
- NPI Registry
- PECOS Enrollment Checklists
Medicare Interactive Medicare answers at your fingertips -->
Eligibility for dme coverage, durable medical equipment (dme).
You must be logged in to bookmark pages.
Email Address * Required
Password * Required
Lost your password?
Whether you have Original Medicare or a Medicare Advantage Plan, Medicare covers your durable medical equipment (DME) if you meet the following two conditions:
- You need the requested DME to help a medical condition or injury
- The equipment is for home use
- Your face-to-face visit, when required, must take place no more than six months before the prescription is written. Your provider should know if Medicare requires a face-to-face visit for the item you need.
- Once you have your PCP’s order or prescription, you must take it to the right supplier to get coverage. Be sure only to use suppliers with approval from Original Medicare or your Medicare Advantage Plan.
Note: There is a different process if you need coverage for a manual or power wheelchair or scooter.
Update your browser to view this website correctly. Update my browser now

- General Dermatology
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Psoriatic Arthritis
- Atopic Dermatitis
- Skin Cancer
- Hidradenitis Suppurativa
- Pigmentary Disorders
- Pediatric Dermatology
- Practice Management
19 tips to prepare for a Medicare audit and site visit
A letter comes across your office fax machine indicating that your practice has been scheduled for an audit and site visit from the Centers for Medicare and Medicaid Services, a Medicare administrative contractor, or a zone program integrity contractor the next morning at 8 a.m. Sound far-fetched? This exact scenario is likely if your practice is scheduled for such a visit from the federal government or a government contractor. The timing is intended to give you little chance to prepare.

The Cutaneous Connection: Make Data-Driven Decisions For Personalized AD Treatment

Tristan Hasbargen, PA-C: Closure Types in Mohs Surgery and More

ReV Up Your Vitiligo Treatment Strategies

QUIZ: Test Your Knowledge of Rosacea Treatment Modalities and the Therapeutic Landscape
European Union Approves Bimekizumab to Treat HS
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


- CMS Site Visits: DME Providers and Suppliers Enrollment Screening
- Durable Medical Equipment Billing
- June 6, 2018
- Martin Jacob

The CMS site visit verification process is a screening mechanism to prevent questionable providers and suppliers from enrolling in Medicare. Providers and suppliers will be screened by the Centers of Medicare & Medicaid Services (CMS) designated national site visit contractor, with the exception of Durable Medical Equipment suppliers which are handled by the National Supplier Clearinghouse .
CMS is strengthening strategies designed to reinforce provider screening activities by increasing site visits to Medicare-enrolled providers and suppliers, enhancing and improving information technology (IT) systems, and implementing continuous data monitoring practices to help make sure practice location data is accurate and in compliance with enrollment requirements.
One important step in Medicare provider enrollment is a site visit.
CMS Site Visits
CMS has the authority to perform site visits on all providers. The site visits verify practice location information to determine compliance with enrollment requirements, and are required for moderate to high-risk providers during initial enrollment, revalidation and when a new location is added. Note that these verification visits are separate from any site visits required at the state level.
The visit is an external and/or internal review by an inspector, with limited disruption to your business. The inspector will introduce himself with a photo ID and a letter of authorization issued and signed by CMS and will:
- Take photos of the business.
- Observe that the business is in operation at that location.
- Verify that the facility is open and operational with both business personnel and customers present.
National Supplier Clearinghouse Site Visits for Durable Medical Equipment Suppliers
DME supplier enrollment site visits are required for initial enrollments and for revalidations.
The inspector will have photo ID, a letter stating the reason for the visit from the inspection manager and a site visit acknowledgement form.
During the visit, the inspector will take photos of your business and conduct an internal review to verify:
- Hours of operation
- Licenses and certifications
- Patient records
- Proof of business records such as rental agreements
Staff interviews may be conducted as well.
It’s important to give site inspectors your full cooperation and to answer their questions fully. Failure to do so, or to meet any verification requirements, may result in denial of your enrollment application and/or revocation of your Medicare billing privileges.
If you require medical credentialing and payer enrollment needs for your practice or medical facility, Our experienced and dedicated specialists will provide all credentialing and enrollment services quickly.
Contact us +1(302) 613-1356 or Schedule a Strategy Meeting
Leave A Comment Cancel Comment
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Dept. of Health & Human Services
National Site Visit Verification (NSV) Initiative
This MLN Matters® Special Edition Article is intended for all providers and suppliers, that enroll in the Medicare program and submit fee-for-service (FFS) claims to Medicare Administrative Contractors (MACs), including home health and hospice MACs, for services provided to Medicare beneficiaries.
Download the Guidance Document
Issued by: Centers for Medicare & Medicaid Services (CMS)
Issue Date: August 11, 2015
DISCLAIMER: The contents of this database lack the force and effect of law, except as authorized by law (including Medicare Advantage Rate Announcements and Advance Notices) or as specifically incorporated into a contract. The Department may not cite, use, or rely on any guidance that is not posted on the guidance repository, except to establish historical facts.
Welcome to the new National Provider Enrollment (NPE) West
The Centers for Medicare and Medicaid Services (CMS) has awarded Palmetto GBA the NPE West contract. Effective November 7, 2022, this contract, along with the NPE East contract, awarded to Novitas Solutions, will replace the current National Supplier Clearinghouse (NSC).
Until November 6, 2022, please continue to work with the current NSC. All enrollment activities will continue as usual until the transition date. The NSC website is still operational and all tools and other information is available.
What's Changing?
Effective November 7, 2022, DMEPOS activities for suppliers located east of the Mississippi River will be administered by Novitas Solutions. DMEPOS activities for suppliers located west of the Mississippi River will continue to be administered by Palmetto GBA.
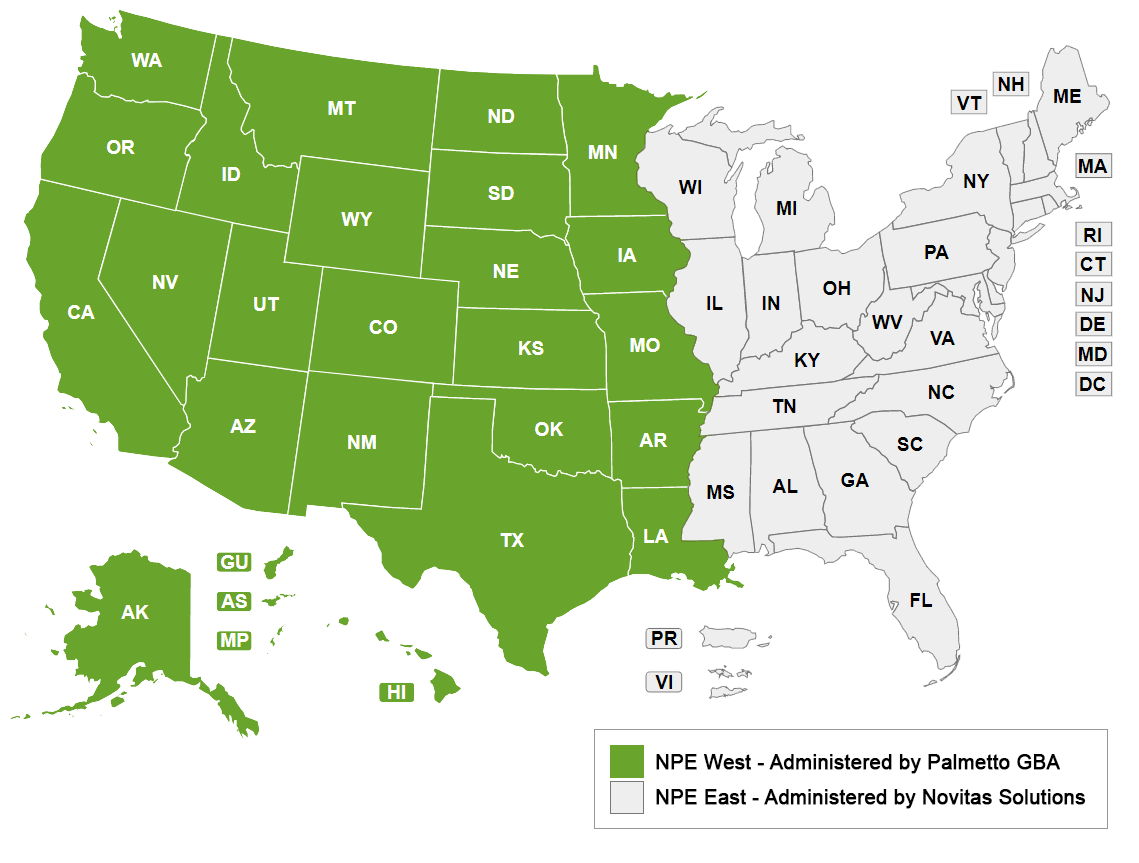
What's Not Changing?
Much will stay the same for NPE West suppliers after the transition.
- The contact information for NPE West suppliers will not change from the current NSC information. Suppliers will continue to call the same customer service number (866-238-9652) and use the existing mailing addresses that exist today.
- All web tools, FAQs, articles and other information on the current NSC website will be adjusted to reflect the new NPE West, but will operationally remain the same.
- Enrollment processes and procedures will continue as currently performed. No change to the enrollment process will occur due to the transition.
COVID-19 Waivers Have Ended
Information that relates to COVID-19 and the Public Health Emergency (PHE). Learn More

Recent News
- Why 1ˢᵗ Credentialing? + –
- Leadership + –
- Testimonials + –
- Client List + –
- Applications + –
- Maintenance + –
- 1stCred + –
Who We Serve
- Behavioral and Mental Health Facilities + –
- Hospitals + –
- Cardiology + –
- Eye Care + –
- Orthopedics + –
- Podiatry + –
- Multispecialty Groups + –
- Partnerships + –
- Telemedicine + –
- Our Blog + –
- Newsletters + –
- Videos + –
- Contact + –
4 Common Mistakes Not to Make During CMS Medicare Verification Site Visits

Practices and businesses who wish to be enrolled as Medicare providers or suppliers must submit to a site visit as part of the provider enrollment or verification process . The Centers of Medicare & Medicaid Services (CMS) use the information obtained during a site visit to verify that your business is legitimate, and that the information submitted to CMS systems for Medicare enrollment is accurate.
An onsite CMS visit is nothing to be concerned about if your legal health care business is fully compliant with Medicare requirements. Although you still want to dot your i’s and cross your t’s by ensuring your ability to provide services to Medicare beneficiaries are secure.
Below are four common errors to avoid:
Having incorrect information on file for each of your practice locations. Accurate and complete information regarding your location(s) must be on file with your Medicare enrollment contractor. If the CMS auditor can’t find your business that’s a problem.
Failing to make updates regarding changes to your practice. If your address or telephone number changes or you modify the hours of operation, you must inform your Medicare enrollment contractor.
Lack of adequate signage for your practice. CMS site visits take place during posted hours or on Monday through Friday between 9:00 a.m. and 5:00 p.m. If the auditor arrives during the hours shown on your sign and no one is there he can record your business as not operational. The same is true if the auditor is unable to locate your business because the sign is not in plain view or doesn’t clearly show the name of your practice.
Refusing to cooperate with a CMS auditor. Verification and reverification requests may entail the auditor taking photos, interviewing staff and asking questions. Medicare providers and suppliers who don’t fully comply with auditor requests or fail to meet any verification requirements may have their enrollment application denied and/or have Medicare billing privileges revoked.
Let Us Manage All Your Payer Enrollment Services
If you require medical credentialing and payer enrollment needs for your practice or medical facility, please contact 1st Assistant. Our experienced and dedicated specialists will provide all credentialing and enrollment services quickly and will monitor your account for ongoing updates and re-attestations. Heidi Henderson , our company owner and President, is eager to meet you and discuss your payer enrollment needs. Please call us at 512.201.2668 or contact us via the website .

Categories: Credentialing Requirement , Knowledge Center
Tags: CMS , medical credentialing , medicare providers , payer enrollment , provider enrollment , verification process
Free Consultation
Thank you for your interest in our services. Let’s schedule a call to talk more about your project and how 1ˢᵗ Credentialing can help. Click here to book an appointment.
Book an appointment
Schedule your free consultation
Our credentialing experts are here to help you assess exactly which solutions you need to put you on the right track. 1ˢᵗ Credentialing includes payor enrollment for all insurance networks. Don’t wait another minute, contact our team today!
Call us at (512) 201-2668 or email us at [email protected]
Stay In the Know
Find out how we can save your time and money by scheduling your free consultation.
Schedule with Calendly
*Your information may be shared with 1st Credentialing’s trusted partners.
- Federal Health News
- Thoughts On Innovation
- Centers for Medicare & Medicaid Services Xtra
- Health and Human Services Xtra
- Military Health System Xtra
- Veterans Affairs Xtra
- Listen + Watch
- FHIT Magazine – Winter 2023 Issue
- FHIT Magazine – Winter 2022 Issue
- FHIT Magazine – Winter 2021 Issue
- FHIT Magazine – Winter 2020 Issue
- FHIT Magazine – Winter 2019 Issue
- FHIT Magazine – Winter 2018 Issue
- FHIT Magazine – Summer 2017 Issue
- FHIT Magazine – Winter 2017 Issue
- FHIT Magazine – Summer 2016 Issue
- Forum Events

CMS awards extension to National Site Visit Contractor
Notice ID: GS-15F-0059MHHSM-500-2012-00009G
Contract: GS-15F-0059M
The Centers for Medicare & Medicaid Services (CMS) implemented certain provisions of the Affordable Care Act to establish additional screening requirements for providers/suppliers.
The CMS Rule 6028-FC published February 2, 2011 (http://federalregister.gov/a/2011-1686) which is now implemented in 42 CFR 424.510, 424.517, 424.530 and 424.535 improves screening mechanisms to prevent questionable providers/suppliers from enrolling in the Medicare program, and requires scheduled, unscheduled or unannounced site visits to providers/suppliers. In addition to fulfilling regulatory requirements, the site verification initiative will also continue to address and support other collaborative efforts with the Office of Inspector General (OIG) and other CMS program integrity initiatives.
The site visit verification process is a screening mechanism to prevent questionable providers/suppliers from enrolling in Part A and Part B of the Medicare program. The initiative described in the SOW builds upon existing site visit programs to create a more efficient, effective, national program to respond to the provisions of the Affordable Care Act, as well as meeting the site-visit requirements described in CMS Publication 100-08, chapter 10 and 15, pertaining to independent diagnostic testing facilities (IDTFs).
Securitas (formerly MSM Security Services, LLC) has been performing as the National Site Visit Contractor (NSVC) for Medicare Parts A and B for 8 years. Previous Limited Source Justifications (LSJs) were executed to maintain continuity while a procurement for multiple award indefinite delivery, indefinite quantity (MA-IDIQ) contracts for Provider Enrollment and Oversight (PEO) services, including two initial task orders for site visit services, was underway. Award of the MA-IDIQ contracts and two task orders for site visit services were completed in August 2020, however, in response to the award, multiple protests were filed with the Government Accountability Office (GAO), necessitating that CMS issue stop work orders for the site verification services task orders. Because on-site visits are required for not only the entire continental United States (U.S.), but also Alaska, Hawaii, and any territories owned by the U.S, Securitas is the only company that is in a position to maintain continuity of site visit services at a reasonable price. Therefore, to maintain continuity of services it is necessary to extend the current task order until the protests are resolved and the work can be transitioned to the new task orders.
Market research was conducted as part of acquisition planning for the PEO MA-IDIQ Contract, which is the vehicle that the new task orders for Site Verification Services (SVS) task orders were awarded under, through a full and open competitive procurement. The current NSVC task order, held by Securitas, was anticipated to run concurrent with the newly awarded task orders to facilitate a transition period. However, a stop work order was issued for the task orders making it necessary for CMS to extend the current GSA task order held by Securitas until the protests can be resolved. Securitas has been providing the services for over eight years and has a record of satisfactory performance as documented via the Contractor Performance Assessment Reporting System (CPARS).
Extension of the current task order is the most suitable and efficient method for maintaining continuity of services. The optional period (January 5, 2021 – May 4, 2021) is included in an attempt to minimize the impact of any delays in resolving the current protests and/or receiving additional protests.
Read more here.
- MSM Security Services
- RELATED ARTICLES
- MORE AUTHORS
- FORUM Editor
- Alexis Keller
- Anthony McCarthy
- Developer Developer
- Jackie Gilbert
- Mary Ann Brown
- Maureen Stiles
- Roxana Pacheco
- Tech Support
- Vicki Barstow
- Julieanne Cooper
- MORE FROM AUTHOR

Zubair Aziz joins McBride Consulting as Senior Director, Public Sector Growth & Strategy

Zscaler seeking Regional Sales Manager – Federal for VA

Zscaler seeking Federal Healthcare Sales Manager for HHS, VA and CMS

Zscaler recruits Tim Hoffman for Regional Sales Manager, Federal
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
FedHealthIT Xtra – Find Out More!
Recent news, impacting the lives of..., making a difference in..., health and human services:..., don’t miss a thing, subscribe to our mailing list.
- Insights NOW
- Listen To FORUM
- FHIT Magazine – Winter 2023 Issue
- Advertise With Us
- Terms of Service and Privacy Policy
© 2023 Forum. All Rights Reserved.
Thanks for visiting! GoodRx is not available outside of the United States. If you are trying to access this site from the United States and believe you have received this message in error, please reach out to [email protected] and let us know.
Getting care in a disaster or emergency
The rules for getting care may change for a short time in areas where one of these has happened:
- The President has declared it an emergency or disaster. Visit the Federal Emergency Management Agency (FEMA) , or call 1-800-621-FEMA (1-800-621-3362) to see if your area is affected (TTY: 1-800-462-7585).
- A governor has declared it an emergency or disaster. Visit your state government's website to find out if your area is affected.
- The Secretary of the Department of Health and Human Service (HHS) has declared a public health emergency. Visit the HHS Public Health Emergency website . Or call us at 1-800-MEDICARE (1-800-633-4227) to find out if your area is affected.
If you have Original Medicare , you can always see any doctor who accepts Medicare. If you have a Medicare Advantage Plan or other Medicare health plan, your plan may make temporary changes to its rules when health plan services get disrupted during an emergency or disaster. Read about how to see your doctor in a disaster or emergency .
Find out what to do if you have a Medicare drug plan and you can’t get to your network pharmacy or you had to leave home without your prescription drugs .
If you're in a Medicare Advantage Plan (Part C) , other Medicare health plan , or Medicare drug plan (Part D) , you're still responsible for paying your premium on time even in an emergency or disaster . Find out how to pay your premium in a disaster or emergency .
Your ESRD Network can help you find facilities that give dialysis services in the area where you're staying temporarily, but if you have a Medicare Advantage Plan or other Medicare health plan, you should check with them first. Find out how to get dialysis in a disaster or emergency.
Find out how to get cancer treatments in a disaster or emergency .
Learn how to replace your Medicare card if it’s damaged or lost.
Find out how to change your address with Medicare.
Find out how to replace lost or damaged durable medical equipment (DME) .

IMAGES
COMMENTS
A site visit helps prevent questionable providers and suppliers from enrolling or staying enrolled in the Medicare Program. The NSVCs conduct unannounced site visits for all Medicare Part A and B providers and suppliers, including DMEPOS suppliers. The NSVCs may conduct an observational site visit or a detailed review to verify enrollment ...
Call nCred today at (423) 443-4525 to discuss your Medicare Provider Enrollment needs. We work with all specialties and have extensive experience processing Medicare applications. From the Medicare Program Integrity Manual: 10.6.20 - Screening: On-site Inspections and Site Verifications (Rev. 11949; Issued: 04-13-23; Effective: 04-21-23 ...
Durable medical equipment (DME) coverage. Medicare Part B (Medical Insurance) covers. medically necessary. DME if your Medicare-enrolled doctor or other health care provider prescribes it for use in your home. DME that Medicare covers includes, but isn't limited to: Blood sugar meters. Blood sugar test strips. Canes.
The site visit verification process is a screening mechanism to prevent questionable providers - and suppliers - from enrolling in Medicare. Providers and suppliers will be screened by the Centers of Medicare & Medicaid Services (CMS)-designated national site visit contractor, with the exception of Durable Medical Equipment suppliers which ...
In December 2006, CMS approved (deemed) 10 ten national accreditation organizations that will accredit suppliers of durable medical equipment, prosthetics, orthotics and supplies (DMEPOS) as meeting new quality standards under Medicare Part B. Most of the accreditation organizations are authorized to accredit all major supplier types, and most ...
Step 3: Complete the Enrollment Application and Electronic Funds Transfer Authorization Agreement CMS-588. Enroll using PECOS, iii the online Medicare enrollment system. PECOS has video and print tutorials and will walk you through your enrollment to ensure your information is accurate. Complete the online PECOS application.
Visit Medicare.gov, or call 1-800-MEDICARE (1-800-633-4227) to get the most current information. TTY users can call 1-877-486-2048. "Medicare Coverage of Durable Medical Equipment & Devices" isn't a legal document. Oficial Medicare Program legal guidance is contained in the relevant statutes, regulations, and rulings.
DME overview. Medicare Part B covers durable medical equipment (DME), which is equipment that serves a medical purpose, is able to withstand repeated use, and is appropriate for use in the home. There are many important things to know about Medicare's coverage rules for DME. Use the information below to learn whether/how you are covered.
Eligibility for DME coverage. Whether you have Original Medicare or a Medicare Advantage Plan, Medicare covers your durable medical equipment (DME) if you meet the following two conditions: Your primary care provider (PCP) must sign an order, prescription, or certificate. In this document, your PCP must state that: Your face-to-face visit, when ...
Suppliers can expect to receive a site visit or survey from the accrediting organization they have applied to as part of the accreditation process. For more information, please visit the MLN Matters fact sheet MLN905710, DMEPOS Accreditation. Once an application is submitted to us, you will have to undergo a site visit conducted by the site ...
If the site visit is set for a branch office, make sure the appropriate administrative personnel and at least one of the physicians who sees Medicare patients are in that office on the day of the site visit. Often, the site visit and audit will be scheduled at a practice's branch office that appears on the claims forms (Form CMS-1500) you ...
The CMS site visit verification process is a screening mechanism to prevent questionable providers and suppliers from enrolling in Medicare. Providers and suppliers will be screened by the Centers of Medicare & Medicaid Services (CMS) designated national site visit contractor, with the exception of Durable Medical Equipment suppliers which are handled by the National Supplier Clearinghouse.
DMEPOS Frequently Asked Questions. 1. Are all DMEPOS suppliers required to be accredited? 2. Are DMEPOS Suppliers required to deliver to the beneficiary? 3. Are suppliers required to have beneficiaries sign a document stating they have received warranty information. Will site inspectors look for this signature? 4.
AO accreditation must indicate the specific products and services for which they're accrediting that supplier to get payment. DMEPOS suppliers must notify their AO when a new DMEPOS location opens. All DMEPOS supplier locations, whether owned or subcontracted, must meet DMEPOS quality standards and get separately accredited to bill us.
National Site Visit Verification (NSV) Initiative. This MLN Matters® Special Edition Article is intended for all providers and suppliers, that enroll in the Medicare program and submit fee-for-service (FFS) claims to Medicare Administrative Contractors (MACs), including home health and hospice MACs, for services provided to Medicare beneficiaries.
The Centers for Medicare and Medicaid Services (CMS) has awarded Palmetto GBA the NPE West contract. Effective November 7, 2022, this contract, along with the NPE East contract, awarded to Novitas Solutions, will replace the current National Supplier Clearinghouse (NSC). Until November 6, 2022, please continue to work with the current NSC.
This face-to-face requirement includes examinations conducted via the Centers for Medicare & Medicaid Services (CMS)-approved use of telehealth examinations (as described in Chapter 15 of the Medicare Benefit Policy Manual and Chapter 12 of the Medicare Claims Processing Manual - CMS Internet-Only Manuals, Publ. 100-02 and 100-04, respectively).
Documentation Checklists. View documentation checklists created to help suppliers ensure all applicable documentation is readily available as part of Medicare claims payment and processing activities. These checklists include the documentation required for payment and retention of that payment in the event of a review by entities looking at ...
Practices and businesses who wish to be enrolled as Medicare providers or suppliers must submit to a site visit as part of the provider enrollment or verification process.The Centers of Medicare & Medicaid Services (CMS) use the information obtained during a site visit to verify that your business is legitimate, and that the information submitted to CMS systems for Medicare enrollment is accurate.
CMS awards extension to National Site Visit Contractor. Oct 1, 2020. By Jackie Gilbert. Notice ID: GS-15F-0059MHHSM-500-2012-00009G. Contract: GS-15F-0059M. The Centers for Medicare & Medicaid Services (CMS) implemented certain provisions of the Affordable Care Act to establish additional screening requirements for providers/suppliers.
All Medicare Round 2021 Durable Medical Equipment, Prosthetics, Orthotics, & Supplies (DMEPOS) Competitive Bidding Program (CBP) Contracts for Off-the-Shelf (OTS) back braces and OTS knee braces expired on December 31, 2023. As of January 1, 2024, there's a temporary gap in the DMEPOS CBP. The Centers for Medicare & Medicaid Services plans to ...
In general, Medicare Part B covers outpatient care and DME, including mobility scooters. To get coverage for a mobility scooter, the scooter must be considered medically necessary and prescribed for use in your home.. You have to get a prescription within 45 days of your in-person evaluation. Medicare typically covers one type of mobility aid for use at home, so you can either get a power ...
Visit the HHS Public Health Emergency website. Or call us at 1-800-MEDICARE (1-800-633-4227) to find out if your area is affected. Learn how to find out if you live in an area that's been declared an emergency or disaster. The usual rules for your medical care may change for a short time if you live in an affected area.
These unsolicited revalidations will be returned. You're required to revalidate—or renew—your enrollment record periodically to maintain Medicare billing privileges. In general, providers and suppliers revalidate every five years but DMEPOS suppliers revalidate every three years. CMS also reserves the right to request off-cycle revalidations.
Posted April 11, 2024. The following tables identify changes to Level II Healthcare Common Procedure Coding System (HCPCS) codes for April 2024. The tables contain only HCPCS codes applicable to items within Medicare DME MAC jurisdiction. There may be other HCPCS code changes for items under the jurisdiction of other Medicare contractors.
initial enrollment, revalidation, adding a new location CMS has the authority to perform site visits on all providers. address validation errors, CAPs/reconsiderations, provider enrollment initiatives. Verifies practice location information to determine compliance with enrollment requirements and supplier standards (IDTF, DME)