
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

14.5: Energy Transported by a Wave
- Last updated
- Save as PDF
- Page ID 19465
In this section, we examine how to model the energy that is transported by waves. Although no material moves along with a wave, mechanical energy can be transported by a wave, as evidenced by the damage caused by the waves from an earthquake.
A wave as being made of simple harmonic oscillators
Consider a wave that is propagating through a medium. We can model the motion of one of the particles in the medium as if it were the motion of a simple harmonic oscillator 1 . This is illustrated in Figure \(\PageIndex{1}\), which shows the displacement as a function of time for a point in the medium located at the origin when a wave passes through that point. The displacement of that point, at \(x=0\) , if we choose \(\phi=0\) , is given by:
\[\begin{aligned} D(x=0,t) = A\sin(-\omega t)\end{aligned}\]
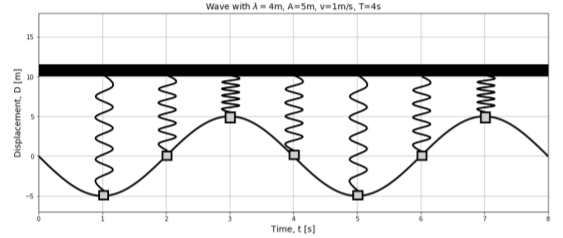
The displacement of the particle in the medium is described by the same equation as the position of a simple harmonic oscillator, with the same angular frequency \(\omega\) , as that of the wave.
We can also view a snapshot of the wave in time, and model the different points in the medium as different oscillators that all have different displacements. This is shown in Figure \(\PageIndex{2}\).
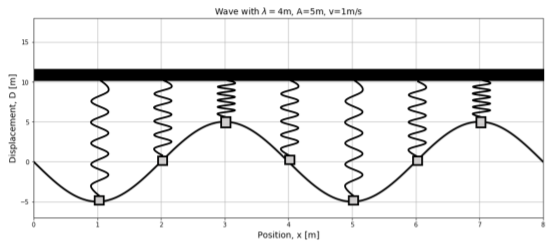
Energy transported in a one dimensional wave
In this section, we show how to describe the energy transported by a one-dimensional wave along a rope. We model each particle in the rope through which the wave propagates as a small simple harmonic oscillator with mass \(m\) , attached to a spring with an effective spring constant, \(k_s\) 2 .
Of course, there is no actual spring, but we can still determine an effective spring constant, \(k_s\) , from the angular frequency:
\[\begin{aligned} \omega &= \sqrt{\frac{k_s}{m}}\\[4pt] \therefore k_s &= \omega^2 m\end{aligned}\]
which corresponds to the spring constant that would give the correct angular frequency for the particle of mass \(m\) .
The total mechanical energy of one oscillator, \(E_m\) , can be evaluated when the oscillator is at its maximal displacement, \(A\) , from its equilibrium, where its kinetic energy is zero:
\[\begin{aligned} E_m = \frac{1}{2}k_s A^2 = \frac{1}{2}\omega^2 m A^2\end{aligned}\]
If the rope is infinitely long, and carries a continuous wave, it will have an infinite amount of energy, as it will correspond to an infinite number of oscillators. Instead, let us calculate how much energy, \(E_\lambda\) , is stored in the wave over one wavelength, \(\lambda\) . To do so, we need to evaluate how many effective oscillators are contained in the rope, over a distance \(\lambda\) , so that we can sum all of their energies together to obtain the energy stored in one wavelength:
\[\begin{aligned} E_\lambda = \sum \frac{1}{2}\omega^2 m A^2\end{aligned}\]
where the sum is over the number of oscillators in one wavelength. Of course, the rope is not actually made of oscillators, but we can model each section of rope of length \(dx\) has being an oscillator of mass \(dm=\mu dx\) , where \(\mu\) is the linear mass density of the rope. The sum (integral) of the energy of the oscillators over one wavelength can thus be written as:
\[\begin{aligned} E_\lambda = \int_0^\lambda \frac{1}{2}\omega^2 \mu A^2 dx = \frac{1}{2}\omega^2 \mu A^2 \lambda\end{aligned}\]
The energy stored in one wavelength is not a very useful property of a wave, since the total energy in the wave depends on the length of the wave. We can describe the rate at which energy is transmitted by the wave (its power), since we know how long, \(T\) , it will take the wave to travel one wavelength, and we just determined how much energy is stored in one wavelength. The average power with which energy is transported by a wave is given by:
\[\begin{aligned} P = \frac{E_\lambda}{T} = \frac{\frac{1}{2}\omega^2 \mu A^2 \lambda}{T}=\frac{1}{2}\omega^2 \mu A^2 v\end{aligned}\]
where \(T\) is the period of the wave, and \(v=\lambda/T\) is the speed of the wave. The power transmitted by a wave on a rope is thus given by:
\[P=\frac{1}{2}\omega ^{2}\mu A^{2}v\]
We can see that the power transmitted by a wave goes as the amplitude, \(A\) , of the wave squared. It thus takes four times more energy to double the amplitude of waves that are sent down a rope.
Energy transported in a spherical, three-dimensional, wave
In this section, we show how to model the rate at which energy is transported in spherical three-dimensional waves, such as the sound waves that are generated when you clap your hands. A spherical sound wave is a pressure disturbance in the air that propagates spherically outwards from a point of emission. We can think of thin spherical shells containing air that expand and contract about their equilibrium position as the wave moves through the shells. The motion of each shell is similar to that of a simple harmonic oscillator of mass \(dm\) , where \(dm\) is the mass of air in the oscillating shell.
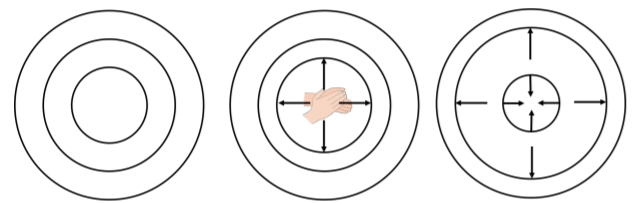
Consider a shell at a radial position, \(r\) , from the source, with thickness \(dr\) , and mass \(dm\) :
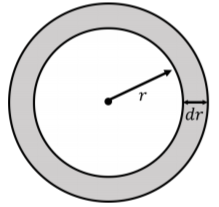
If the medium has a density, \(\rho\) , then the mass of the shell is given by:
\[\begin{aligned} dm = \rho dV = \rho 4\pi r^2 dr\end{aligned}\]
where \(dV = 4\pi r^2 dr\) is the volume of the shell. Again, if we model each shell as a simple harmonic oscillator with mass \(dm\) , then the energy, \(dE\) , stored in that oscillating shell is given by:
\[\begin{aligned} dE = \frac{1}{2}k_s A^2 = \frac{1}{2}\omega^2 dm A^2 = \frac{1}{2}\omega^2 A^2 \rho 4\pi r^2 dr=2\pi\rho \omega^2 A^2 r^2 dr\end{aligned}\]
where \(\omega\) is the angular frequency of the wave, and \(A\) is the amplitude of the wave. We expressed the effective spring constant, \(k_s\) , in terms of the angular frequency of the simple harmonic oscillator and its mass, as we did in the previous section. It now makes less sense to determine the energy that is stored in one wavelength of the wave because the energy, \(dE\) , stored in one shell depends on the location, \(r\) , of that shell. This was not the case for a one-dimensional wave, where the energy stored in one oscillator did not depend on the position of that oscillator.
The rate at which energy is transported by the wave is given by:
\[\begin{aligned} P = \frac{dE}{dt}\end{aligned}\]
We can use the Chain Rule to change this into a derivative over \(r\) :
\[\begin{aligned} P = \frac{dE}{dr}\frac{dr}{dt}=\frac{dE}{dr}v\end{aligned}\]
where \(\frac{dr}{dt}=v\) is the speed of the wave (the rate of change of the radius of a shell). The power transmitted by the spherical wave is thus given by:
\[\begin{aligned} P &=\frac{dE}{dr}v =2\pi\rho \omega^2 A^2 r^2 v\end{aligned}\]
where the power appears to depends on how far you are from the source ( \(r\) ).
Suppose that you have a \(50\text{W}\) speaker emitting sound; each radial shell emanating from the speaker must transport energy at a rate of \(50\text{W}\) . This is simply a statement that the energy radiated by the speaker has to move from one shell to the next and be conserved. Since the power transported by a shell appears to depend on the radius of the shell, if the power transmitted by each shell is the same, then the amplitude of the wave in each shell must decrease, so that the power does not actually depend on the radius of the shell. In particular, for a spherical wave, the amplitude will decrease as a function of distance from the source:
\[\begin{aligned} P& = \text{constant}\\[4pt] \therefore A&=\frac{1}{r}\sqrt{\frac{P}{2\pi\rho \omega^2 v}}\end{aligned}\]
This is very different from the propagation of a one-dimensional wave, in which the amplitude does not change with distance. In practice, if there are energy losses due to, say, friction, then the amplitude of a one-dimensional wave would also decrease with distance from the source, but this is a different effect.
Olivia's Thoughts
Here’s a slightly different way to think about why the amplitude of the wave decreases as you get further from the source. When a spherical wave travels outwards, energy is passed from one shell to the next. The outer shells are bigger than the inner shells, and so they will contain more particles. Because of conservation of energy, when the energy is transferred from one shell to the next, the total energy stays the same. In the outer shells, the energy must be shared between a greater number of particles, so each particle gets less energy, and therefore oscillates with a smaller amplitude than the particles in the previous shell did.
To remember this, imagine the shells in Figure \(\PageIndex{3}\) are circles of kids standing side by side. The innermost circle has \(10\) kids and the outermost circle has \(100\) kids. If you have \(100\) candies, and you give them to the kids in the innermost circle, each will get \(10\) so they will get really hyper and start jumping around a lot. If you instead give the \(100\) candies to the kids in the outermost circle, each will only get one. The kids will only get a little bit hyper and jump around less.
The “intensity of a wave”, \(I\) , is defined as the power per unit area that is transmitted by the wave. For a spherical wave front at radial position \(r\) , with area \(4\pi r^2\) , the intensity of the wave is defined as:
\[\begin{aligned} I = \frac{P}{4\pi r^2} = \frac{1}{2}\rho \omega^2 A^2 v\end{aligned}\]
Usually, the intensity of a wave is something that you can measure, as it corresponds to the power delivered into some measuring device with a known surface area. For example, we cannot directly measure the total power that is transported by the waves from an earthquake, as we would need an instrument that could encompass the entire resulting wave. Instead, we can measure the intensity of waves from the earthquake by measuring how much power is delivered into some instrument with a known surface area. By knowing our distance from the earthquake, we could then determine the total power output of the earthquake.
The intensity is a measure of how much energy is delivered per unit area by a wave and goes down as the square of the distance from the source (since \(A\propto 1/r\) ). If the source of the wave is an earthquake, then your house will have four times less damage than your friend’s, if your house is located only twice as far from the epicenter as your friend’s. You will cause four times less damage to your ears if you move only twice as far away from the stage at a rock concert.
1. If the medium has a linear restoring force or if the amplitude of the oscillations is small.
2. We use \(k_{s}\) for the spring constant, to distinguish it from \(k\), the wave number.

Anatomy of an Electromagnetic Wave
Energy, a measure of the ability to do work, comes in many forms and can transform from one type to another. Examples of stored or potential energy include batteries and water behind a dam. Objects in motion are examples of kinetic energy. Charged particles—such as electrons and protons—create electromagnetic fields when they move, and these fields transport the type of energy we call electromagnetic radiation, or light.

What are Electromagnetic and Mechanical waves?
Mechanical waves and electromagnetic waves are two important ways that energy is transported in the world around us. Waves in water and sound waves in air are two examples of mechanical waves. Mechanical waves are caused by a disturbance or vibration in matter, whether solid, gas, liquid, or plasma. Matter that waves are traveling through is called a medium. Water waves are formed by vibrations in a liquid and sound waves are formed by vibrations in a gas (air). These mechanical waves travel through a medium by causing the molecules to bump into each other, like falling dominoes transferring energy from one to the next. Sound waves cannot travel in the vacuum of space because there is no medium to transmit these mechanical waves.
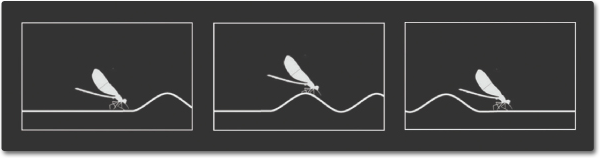
ELECTROMAGNETIC WAVES
Electricity can be static, like the energy that can make your hair stand on end. Magnetism can also be static, as it is in a refrigerator magnet. A changing magnetic field will induce a changing electric field and vice-versa—the two are linked. These changing fields form electromagnetic waves. Electromagnetic waves differ from mechanical waves in that they do not require a medium to propagate. This means that electromagnetic waves can travel not only through air and solid materials, but also through the vacuum of space.
In the 1860's and 1870's, a Scottish scientist named James Clerk Maxwell developed a scientific theory to explain electromagnetic waves. He noticed that electrical fields and magnetic fields can couple together to form electromagnetic waves. He summarized this relationship between electricity and magnetism into what are now referred to as "Maxwell's Equations."
Heinrich Hertz, a German physicist, applied Maxwell's theories to the production and reception of radio waves. The unit of frequency of a radio wave -- one cycle per second -- is named the hertz, in honor of Heinrich Hertz.
His experiment with radio waves solved two problems. First, he had demonstrated in the concrete, what Maxwell had only theorized — that the velocity of radio waves was equal to the velocity of light! This proved that radio waves were a form of light! Second, Hertz found out how to make the electric and magnetic fields detach themselves from wires and go free as Maxwell's waves — electromagnetic waves.
WAVES OR PARTICLES? YES!
Light is made of discrete packets of energy called photons. Photons carry momentum, have no mass, and travel at the speed of light. All light has both particle-like and wave-like properties. How an instrument is designed to sense the light influences which of these properties are observed. An instrument that diffracts light into a spectrum for analysis is an example of observing the wave-like property of light. The particle-like nature of light is observed by detectors used in digital cameras—individual photons liberate electrons that are used for the detection and storage of the image data.
POLARIZATION
One of the physical properties of light is that it can be polarized. Polarization is a measurement of the electromagnetic field's alignment. In the figure above, the electric field (in red) is vertically polarized. Think of a throwing a Frisbee at a picket fence. In one orientation it will pass through, in another it will be rejected. This is similar to how sunglasses are able to eliminate glare by absorbing the polarized portion of the light.
DESCRIBING ELECTROMAGNETIC ENERGY
The terms light, electromagnetic waves, and radiation all refer to the same physical phenomenon: electromagnetic energy. This energy can be described by frequency, wavelength, or energy. All three are related mathematically such that if you know one, you can calculate the other two. Radio and microwaves are usually described in terms of frequency (Hertz), infrared and visible light in terms of wavelength (meters), and x-rays and gamma rays in terms of energy (electron volts). This is a scientific convention that allows the convenient use of units that have numbers that are neither too large nor too small.
The number of crests that pass a given point within one second is described as the frequency of the wave. One wave—or cycle—per second is called a Hertz (Hz), after Heinrich Hertz who established the existence of radio waves. A wave with two cycles that pass a point in one second has a frequency of 2 Hz.
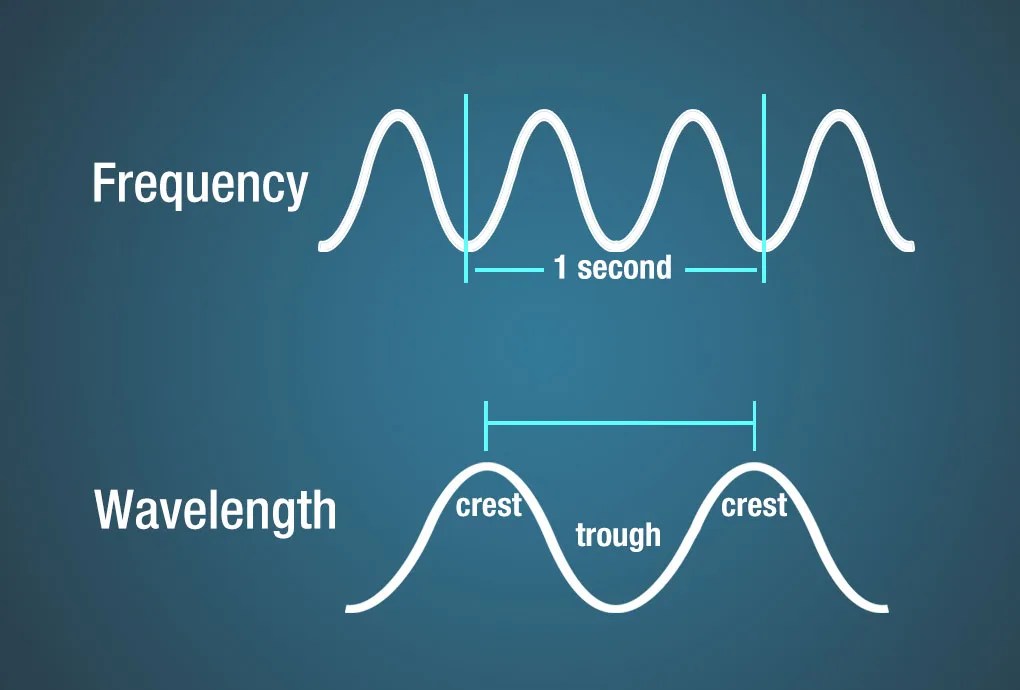
Electromagnetic waves have crests and troughs similar to those of ocean waves. The distance between crests is the wavelength. The shortest wavelengths are just fractions of the size of an atom, while the longest wavelengths scientists currently study can be larger than the diameter of our planet!
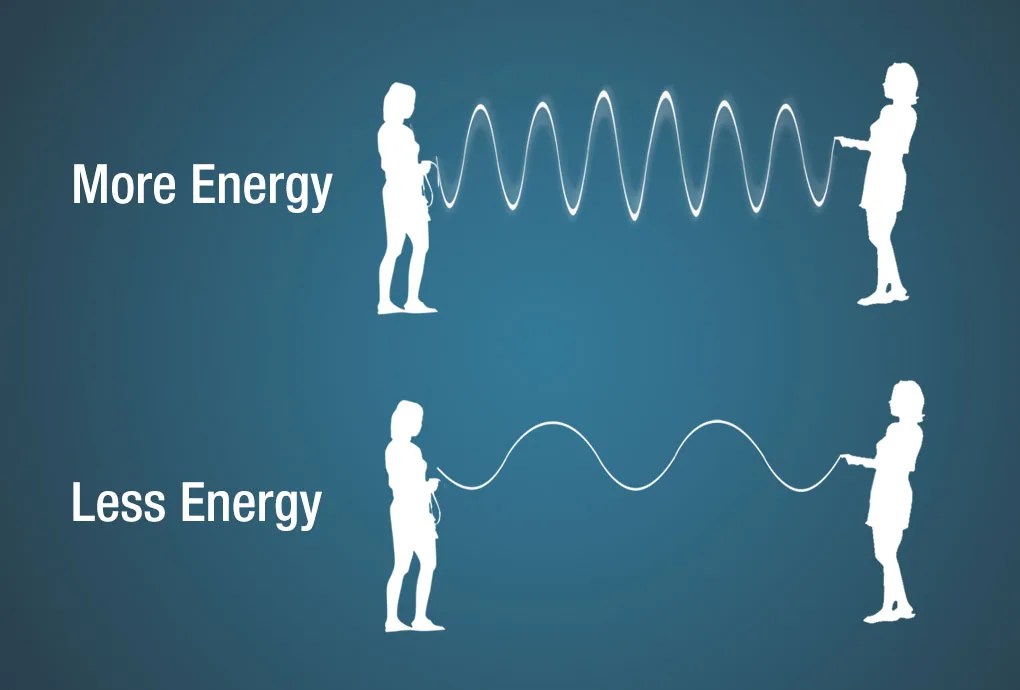
An electromagnetic wave can also be described in terms of its energy—in units of measure called electron volts (eV). An electron volt is the amount of kinetic energy needed to move an electron through one volt potential. Moving along the spectrum from long to short wavelengths, energy increases as the wavelength shortens. Consider a jump rope with its ends being pulled up and down. More energy is needed to make the rope have more waves.
Next: Wave Behaviors
National Aeronautics and Space Administration, Science Mission Directorate. (2010). Anatomy of an Electromagnetic Wave. Retrieved [insert date - e.g. August 10, 2016] , from NASA Science website: http://science.nasa.gov/ems/02_anatomy
Science Mission Directorate. "Anatomy of an Electromagnetic Wave" NASA Science . 2010. National Aeronautics and Space Administration. [insert date - e.g. 10 Aug. 2016] http://science.nasa.gov/ems/02_anatomy
Discover More Topics From NASA
James Webb Space Telescope

Perseverance Rover

Parker Solar Probe

NOTIFICATIONS
Waves as energy transfer.
- + Create new collection
‘Wave’ is a common term for a number of different ways in which energy is transferred:
- In electromagnetic waves, energy is transferred through vibrations of electric and magnetic fields.
- In sound waves, energy is transferred through vibration of air particles or particles of a solid through which the sound travels.
- In water waves, energy is transferred through the vibration of the water particles.
Waves transfer energy but not mass
When we watch surf waves coming into shore, it’s easy to think that individual water particles are moving towards us, but that’s not actually the case. The particles involved in waves move back and forth perpendicularly to the way the wave is going, but don’t move significantly in the direction of the wave. The particles ‘take part’ in the wave by bumping into one another and transferring energy. This is why energy can be transferred, even though the average position of the particles doesn’t change.
How does this work? It can help to think of a buoy bobbing in the ocean. The buoy is moved up and down by the waves that pass by it, but doesn’t move directionally across the water.
You could also think about a Mexican wave at a sports match. The wave moves around the arena, but the audience members don’t move around with it – they only stand up and sit down (a perpendicular movement to the wave direction).
Particles in a water wave exchange kinetic energy for potential energy
When particles in water become part of a wave, they start to move up or down. This means that kinetic energy (energy of movement) has been transferred to them. As the particles move further away from their normal position (up towards the wave crest or down towards the trough), they slow down. This means that some of their kinetic energy has been converted into potential energy – the energy of particles in a wave oscillates between kinetic and potential energy.
Thinking about potential energy can help us understand why tsunamis can be so damaging. When a tsunami approaches the shore, it shoals (becomes much higher), so the water particles are displaced further from equilibrium. They acquire a lot of potential energy, and this is released when the wave interacts with land.
Measuring the energy in a wave
Why do some waves have more energy than others? A wave’s frequency and wavelength are both indicators of its energy, but this differs for different types for waves.
For water waves, those with a high speed and long wavelength (like a tsunami) have the most energy. For electromagnetic waves, speed is constant, so waves with a high frequency and a short wavelength (like X-rays) are the most energetic.
For all waves, a greater amplitude means more energy.
In the electromagnetic spectrum interactive you can click on various wavelengths to learn more about the waves that make up the spectrum.
Harnessing wave energy
Scientists in New Zealand and elsewhere are looking at how to turn the energy of water waves into electricity. The oceans around New Zealand are promising places to generate wave power because we have large waves and strong currents. Generating wave power would involve an underwater device (like a paddle, for example) that would move in response to waves and drive a turbine that would produce electricity.
The idea of wave power is appealing because waves are a sustainable resource – they can’t be used up (unlike other resources, like coal, that are used for making electricity in New Zealand). However, they are quite inefficient – they need a lot of coastal space to generate useful quantities of energy. Using mathematical modelling and physical model building, Kiwi scientists are investigating how to harness wave power, but it will be some time before we’re using electricity from wave power in our homes.
Between 2007 and 2011 the Energy Efficiency and Conservation Authority (EECA) administered the Marine Energy Deployment Fund which funded marine energy projects. After a review none of the projects were selected to progress further and, as of 2016, EECA believe that the abundance of cheaper renewable energy resources in New Zealand makes it unlikely marine energy will contribute to the national grid in the foreseeable future. Investigations into harnessing the energy of ocean waves continues in other countries.
From 2017 to 2019, as part of a Sustainable Seas Innovation Fund project, NIWA investigated whether generating electricity from the strong tidal currents within the Cook Strait would be viable for Aotearoa. To find out more, see Energy from tidal currents – Kick-starting a new marine industry with huge potential from NIWA's website.
Activity ideas
Use a Mexican wave to demonstrate how waves transfer energy and to help your students visualise the wave behaviours of reflection, constructive interference and shoaling.
Use an interactive or paper-based Venn diagram to illustrate the key similarities and differences between tsunami waves and surf waves .
More on waves
Explore more about waves, such as sound and energy by browsing the resources under our waves concept .
Useful links
In 2021 NIWA ran a webinar: A step closer to a future powered by tidal current energy , in which the results of the Energy from tidal currents project are presented.This project investigated the viability of generation electricity from the strong tidal currents within Cook Strait.
Find out more about using waves as an energy source in this Wikipedia article .
Watch this 2011 video from NIWA: Current conversion – tidal and wave energy in New Zealand .
See our newsletters here .
Would you like to take a short survey?
This survey will open in a new tab and you can fill it out after your visit to the site.
Mechanical Waves: Explanation and Examples
- The Albert Team
- Last Updated On: August 16, 2023
Mechanical waves play a big part in our everyday lives, from the sound of music to the ripples in water. In this post, we’ll explore what mechanical waves are, the different types, and some basic properties like amplitude, frequency, and speed. If you’ve ever wondered if these waves need a medium or how to use the wave equation, you’re in the right place.
What We Review
What is a mechanical wave?
A mechanical wave is a disturbance or oscillation that travels through matter (medium), transferring energy from one point to another. Unlike electromagnetic waves which can travel through a vacuum, mechanical waves rely on particles in a medium to transport their energy.
Mechanical Waves and Matter
At the heart of understanding mechanical waves is recognizing their relationship with matter. When you drop a pebble into a pond, the water’s surface ripples outward. Here, the water particles are not moving outwards with the ripple. Rather, they’re moving up and down, transferring the wave’s energy to neighboring particles. The actual matter (water in this case) doesn’t travel a long distance, but the energy does, in the form of a wave.
Do mechanical waves require a medium?
Yes, mechanical waves do require a medium to travel through. This could be a solid, liquid, or gas. The reason behind this lies in the definition of mechanical waves: they propagate energy by causing particles in the medium to oscillate. Without a medium, there are no particles to move, and therefore, the wave cannot exist. It’s worth noting that this is a key factor between mechanical waves and electromagnetic waves. Electromagnetic waves behave differently and can travel through the vacuum of space.
Types of Mechanical Waves
Mechanical waves come in different shapes and sizes. There are two main types based on how they move:
- Transverse waves: In these waves, particles of the medium move perpendicular to the direction of the wave. Light waves and waves on a string are examples of transverse waves.
- Longitudinal waves: Here, particles of the medium move parallel to the direction of the wave. Sound waves in air are a classic example of longitudinal waves.
Both these types can be seen in our daily lives, from the strings of a musical instrument vibrating to the audible chatter of a busy marketplace. Understanding the nature and mechanics of these waves is the first step to grasping the larger complexities of the physical world.
How to Represent Mechanical Waves
Representing mechanical waves graphically provides us with an insightful way to understand their behavior and characteristics. By plotting them on a graph, we can visualize and analyze their various quantities. Let’s look deeper into these essential quantities to get a holistic understanding.
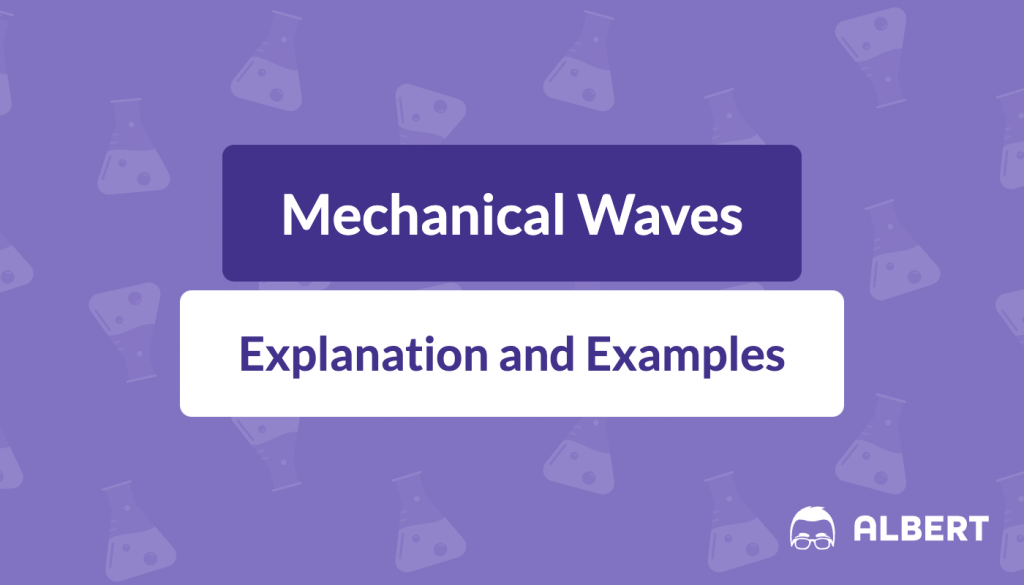
The wavelength of a wave is the distance between two successive points that are in phase. It Is typically measured from one crest to the next or from one trough to the next. It’s denoted by the Greek letter lambda ( \lambda ). The distance between one wave crest and the subsequent wave crest is its wavelength. In the wave pictured below, the wavelength is 2\text{ units} .
In radio broadcasting, different stations transmit on different wavelengths (or frequencies) to avoid interference with each other.
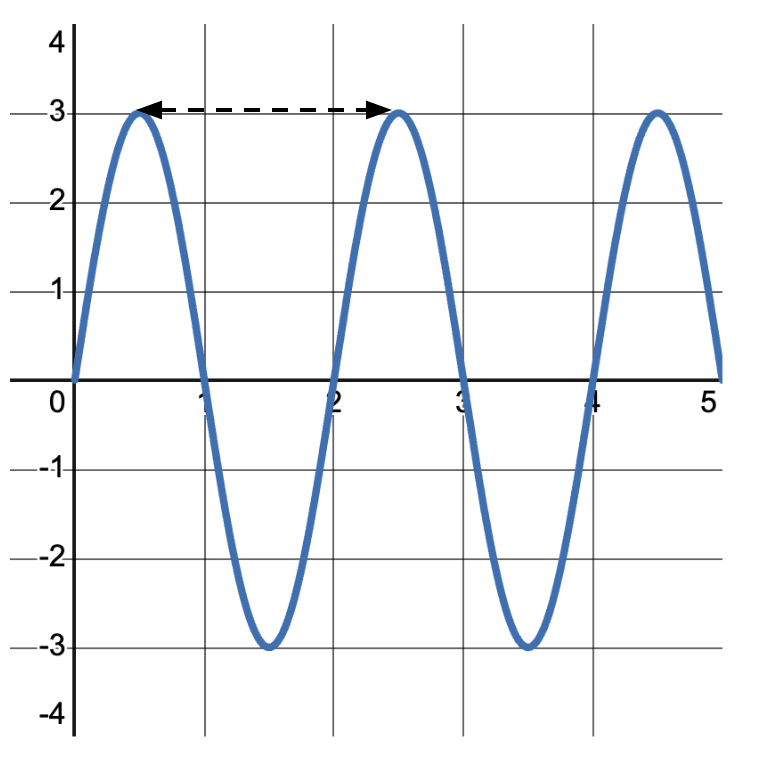
The amplitude of a wave refers to the maximum displacement of particles from their rest or equilibrium position. It’s a measure of the wave’s energy. In the wave pictured below, the amplitude is measured from the center line to the peak. The amplitude of this wave is 3\text{ units} .
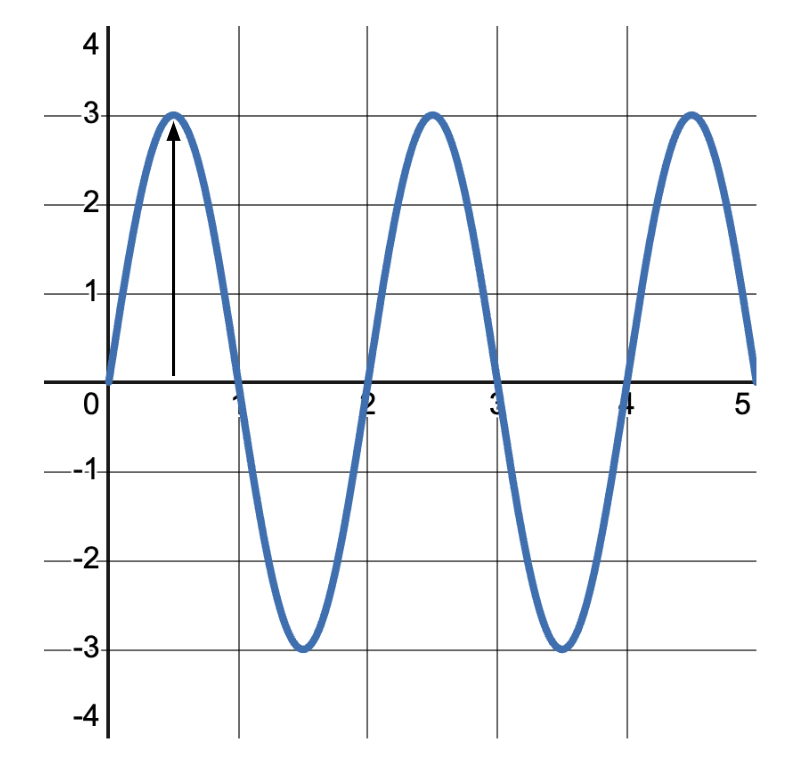
Higher amplitude means more energy, and vice versa. When you throw a larger stone into a pond, the ripples or water waves produced have higher amplitude compared to a smaller stone.
An example of the amplitude of mechanical waves lies in music. The amplitude of sound waves determines the loudness or volume of the sound produced. Higher amplitude results in louder sounds.
Frequency refers to the number of complete cycles or oscillations a wave undergoes in one second. It is measured in Hertz (Hz). For example, when a guitar string is plucked, it vibrates at a certain frequency, producing a specific musical note. The wave in the image below has 2.5\text{ cycles} . If we were told that wave pattern took 1\text{ s} , then the frequency would be 2.5\text{ cycles per second} or 2.5\text{ Hz} .
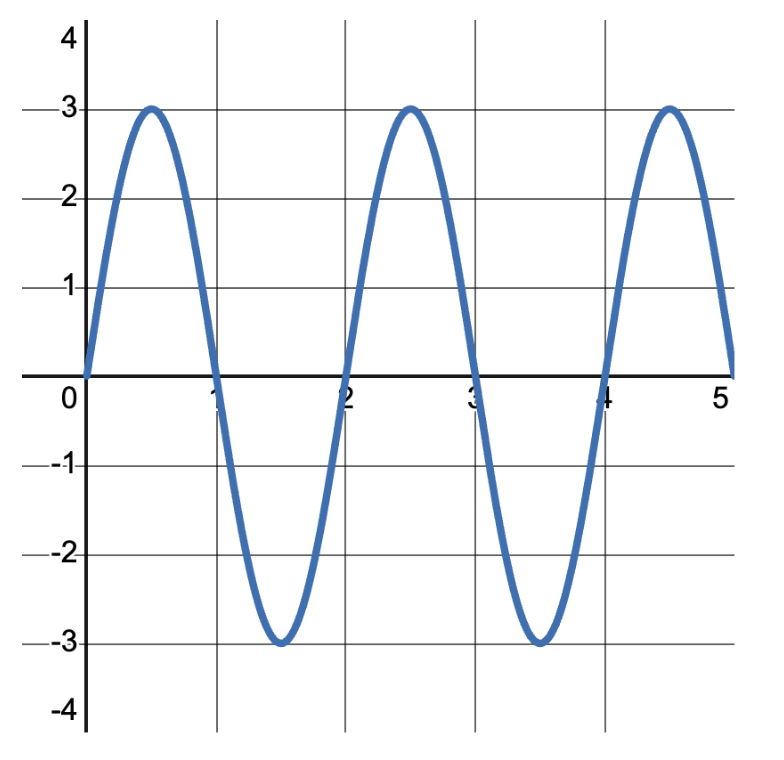
Wave speed is the speed at which a wave travels through a medium. It’s typically calculated by multiplying the frequency of the wave by its wavelength. This is represented in the wave equation as v=f\times \lambda , where v is the wave speed, f is the frequency, and \lambda is the wavelength.
The Wave Equation
The wave equation is a fundamental relationship that helps to describe how waves, including mechanical waves, propagate. It’s a valuable tool for scientists and engineers working with waves in various contexts, from acoustics to optics.
What is the wave equation?
The wave equation represents the relationship between the speed of a wave, its frequency, and its wavelength.
…where:
- v is the wave speed, typically measured in meters per second (m/s).
- f is the frequency of the wave, measured in Hertz (Hz).
- \lambda is the wavelength, which is the distance between successive points in phase, measured in meters (m).
How to use the wave equation
Using the wave equation is relatively straightforward once you have two of the three variables ( v , f , \lambda ). If you know any two, you can easily find the third.
Suppose you have a wave with a frequency of 50\text{ Hz} and a wavelength of 2\text{ meters} . What is its speed?
Using the wave equation:
First, identify what we know:
- f=50\text{ Hz}
- \lambda=2\text{ meters}
- v is unknown
Our equation is already set up to solve for v , we can substitute our values and calculate:
So, the wave travels at a speed of 100 meters per second.
Consider a sound wave traveling at 340\text{ m/s} (the speed of sound in dry air at room temperature) with a frequency of 170\text{ Hz} . What is its wavelength?
- v= 340\text{ m/s}
- f=170\text{ Hz}
- \lambda is unknown
Rearrange the wave equation to solve for \lambda :
Then substitute and solve:
The wavelength of the sound wave is 2 meters.
Mastering the wave equation allows us to analyze and predict wave behavior in diverse settings. It’s used in many applications from designing musical instruments to understanding electromagnetic wave propagation in communication systems.
Mechanical waves, with their ubiquity in our daily lives, often go unnoticed, yet they underpin many phenomena we encounter. From the sound waves that convey conversations and music to the ripples in a pond from a tossed stone, understanding these waves deepens our appreciation for the world around us. This post aimed to shed light on the nature of mechanical waves, their key components, and the mathematical relationships governing them. By grasping concepts like amplitude, frequency, and the wave equation, we can better navigate and innovate in fields ranging from music to telecommunications.
Interested in a school license?
Popular posts.
AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.
Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .
SAT® Score Calculator
See how scores on each section impacts your overall SAT® score
ACT® Score Calculator
See how scores on each section impacts your overall ACT® score
Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
Interested in a school license?

Bring Albert to your school and empower all teachers with the world's best question bank for: ➜ SAT® & ACT® ➜ AP® ➜ ELA, Math, Science, & Social Studies aligned to state standards ➜ State assessments Options for teachers, schools, and districts.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

13.1: Electromagnetic Waves
- Last updated
- Save as PDF
- Page ID 2843

Did you ever wonder how a microwave works? It directs invisible waves of radiation toward the food placed inside of it. The radiation transfers energy to the food, causing it to get warmer. The radiation is in the form of microwaves, which are a type of electromagnetic waves.
What Are Electromagnetic Waves?
Electromagnetic waves are waves that consist of vibrating electric and magnetic fields. Like other waves, electromagnetic waves transfer energy from one place to another. The transfer of energy by electromagnetic waves is called electromagnetic radiation . Electromagnetic waves can transfer energy through matter or across empty space.
Q: How do microwaves transfer energy inside a microwave oven?
A: They transfer energy through the air inside the oven to the food.
May the Force Be With You
A familiar example may help you understand the vibrating electric and magnetic fields that make up electromagnetic waves. Consider a bar magnet, like the one in the Figure below. The magnet exerts magnetic force over an area all around it. This area is called a magnetic field. The field lines in the diagram represent the direction and location of the magnetic force. Because of the field surrounding a magnet, it can exert force on objects without touching them. They just have to be within its magnetic field.

Q: How could you demonstrate that a magnet can exert force on objects without touching them?
A: You could put small objects containing iron, such as paper clips, near a magnet and show that they move toward the magnet.
An electric field is similar to a magnetic field. It is an area of electrical force surrounding a positively or negatively charged particle. You can see electric fields in the following Figure below. Like a magnetic field, an electric field can exert force on objects over a distance without actually touching them.

How an Electromagnetic Wave Begins
An electromagnetic wave begins when an electrically charged particle vibrates. The Figure below shows how this happens. A vibrating charged particle causes the electric field surrounding it to vibrate as well. A vibrating electric field, in turn, creates a vibrating magnetic field. The two types of vibrating fields combine to create an electromagnetic wave.

How an Electromagnetic Wave Travels
As you can see in the Figure above, the electric and magnetic fields that make up an electromagnetic wave are perpendicular (at right angles) to each other. Both fields are also perpendicular to the direction that the wave travels. Therefore, an electromagnetic wave is a transverse wave. However, unlike a mechanical transverse wave, which can only travel through matter, an electromagnetic transverse wave can travel through empty space. When waves travel through matter, they lose some energy to the matter as they pass through it. But when waves travel through space, no energy is lost. Therefore, electromagnetic waves don’t get weaker as they travel. However, the energy is “diluted” as it travels farther from its source because it spreads out over an ever-larger area.
Electromagnetic Wave Interactions
When electromagnetic waves strike matter, they may interact with it in the same ways that mechanical waves interact with matter. Electromagnetic waves may:
- reflect, or bounce back from a surface;
- refract, or bend when entering a new medium;
- diffract, or spread out around obstacles.
Electromagnetic waves may also be absorbed by matter and converted to other forms of energy. Microwaves are a familiar example. When microwaves strike food in a microwave oven, they are absorbed and converted to thermal energy, which heats the food.
Sources of Electromagnetic Waves
The most important source of electromagnetic waves on Earth is the sun. Electromagnetic waves travel from the sun to Earth across space and provide virtually all the energy that supports life on our planet. Many other sources of electromagnetic waves depend on technology. Radio waves, microwaves, and X rays are examples. We use these electromagnetic waves for communications, cooking, medicine, and many other purposes.
Launch the Light Wave simulation below to help you visualize light as a transverse wave moving through the electromagnetic field. Be sure to adjust the wavelength band slider to observe waves of different sizes, such as Radio Waves and X-Rays. There is also an illustration of objects of comparable sizes next to the electromagnetic spectrum to help you imagine the sizes of these invisible light waves.
Interactive Element
- Electromagnetic waves are waves that consist of vibrating electric and magnetic fields. They transfer energy through matter or across space. The transfer of energy by electromagnetic waves is called electromagnetic radiation.
- The electric and magnetic fields of an electromagnetic wave are areas of electric or magnetic force. The fields can exert force over objects at a distance.
- An electromagnetic wave begins when an electrically charged particle vibrates. This causes a vibrating electric field, which in turn creates a vibrating magnetic field. The two vibrating fields together form an electromagnetic wave.
- An electromagnetic wave is a transverse wave that can travel across space as well as through matter. When it travels through space, it doesn’t lose energy to a medium as a mechanical wave does.
- When electromagnetic waves strike matter, they may be reflected, refracted, or diffracted. Or they may be absorbed by matter and converted to other forms of energy.
- The most important source of electromagnetic waves on Earth is the sun. Many other sources of electromagnetic waves depend on technology.
- What is an electromagnetic wave?
- Define electromagnetic radiation.
- Describe the electric and magnetic fields of an electromagnetic wave.
- How does an electromagnetic wave begin? How does it travel?
- Compare and contrast electromagnetic and mechanical transverse waves.
- List three sources of electromagnetic waves on Earth.
Explore More
Watch the electromagnetic wave animation and then answer the questions below.
- Identify the vibrating electric and magnetic fields of the wave.
- Describe the direction in which the wave is traveling.
Additional Resources
Study Guide: Wave Optics Study Guide
Real World Application: Printing with Light, The Incredible Hulk
PLIX: Play, Learn, Interact, eXplore: Energy Levels: Bohr's Atomic Model
Energy Transfers and Transformations
Energy cannot be created or destroyed, but it can be transferred and transformed. There are a number of different ways energy can be changed, such as when potential energy becomes kinetic energy or when one object moves another object.
Earth Science, Physics
Water Boiling Pot
There are three types of thermal energy transfer: conduction, radiation, and convection. Convection is a cyclical process that only occurs in fluids.
Photograph by Liu Kuanxi

Energy cannot be created or destroyed, meaning that the total amount of energy in the universe has always been and will always be constant. However, this does not mean that energy is immutable; it can change form and even transfer between objects. A common example of energy transfer that we see in everyday life is the transfer of kinetic energy —the energy associated with motion—from one moving object to a stationary object via work. In physics, work is a measure of energy transfer and refers to the force applied by an object over a distance. When a golf club is swung and hits a stationary golf ball, some of the club’s kinetic energy transfers to the ball as the club does “work” on the ball. In an energy transfer such as this one, energy moves from one object to another, but stays in the same form. A kinetic energy transfer is easy to observe and understand, but other important transfers are not as easy to visualize. Thermal energy has to do with the internal energy of a system due to its temperature. When a substance is heated, its temperature rises because the molecules it is composed of move faster and gain thermal energy through heat transfer. Temperature is used as a measurement of the degree of “hotness” or “coldness” of an object, and the term heat is used to refer to thermal energy being transferred from a hotter system to a cooler one. Thermal energy transfers occur in three ways: through conduction , convection , and radiation . When thermal energy is transferred between neighboring molecules that are in contact with one another, this is called conduction . If a metal spoon is placed in a pot of boiling water, even the end not touching the water gets very hot. This happens because metal is an efficient conductor , meaning that heat travels through the material with ease. The vibrations of molecules at the end of the spoon touching the water spread throughout the spoon, until all the molecules are vibrating faster (i.e., the whole spoon gets hot). Some materials, such as wood and plastic, are not good conductors —heat does not easily travel through these materials—and are instead known as insulators . Convection only occurs in fluids, such as liquids and gases. When water is boiled on a stove, the water molecules at the bottom of the pot are closest to the heat source and gain thermal energy first. They begin to move faster and spread out, creating a lower density of molecules at the bottom of the pot. These molecules then rise to the top of the pot and are replaced at the bottom by cooler, denser water. The process repeats, creating a current of molecules sinking, heating up, rising, cooling down, and sinking again. The third type of heat transfer— radiation —is critical to life on Earth and is important for heating bodies of water. With radiation , a heat source does not have to touch the object being heated; radiation can transfer heat even through the vacuum of space. Nearly all thermal energy on Earth originates from the sun and radiates to the surface of our planet, traveling in the form of electromagnetic waves, such as visible light. Materials on Earth then absorb these waves to be used for energy or reflect them back into space. In an energy transformation , energy changes form. A ball sitting at the top of a hill has gravitational potential energy , which is an object’s potential to do work due to its position in a gravitational field. Generally speaking, the higher on the hill this ball is, the more gravitational potential energy it has. When a force pushes it down the hill, that potential energy transforms into kinetic energy . The ball continues losing potential energy and gaining kinetic energy until it reaches the bottom of the hill. In a frictionless universe, the ball would continue rolling forever upon reaching the bottom, since it would have only kinetic energy . On Earth, however, the ball stops at the bottom of the hill due to the kinetic energy being transformed into heat by the opposing force of friction. Just as with energy transfers , energy is conserved in transformations. In nature, energy transfers and transformations happen constantly, such as in a coastal dune environment. When thermal energy radiates from the sun, it heats both the land and ocean, but water has a specific high heat capacity, so it heats up slower than land. This temperature difference creates a convection current, which then manifests as wind. This wind possesses kinetic energy , which it can transfer to grains of sand on the beach by carrying them a short distance. If the moving sand hits an obstacle, it stops due to the friction created by the contact and its kinetic energy is then transformed into thermal energy , or heat. Once enough sand builds up over time, these collisions can create sand dunes, and possibly even an entire dune field. These newly formed sand dunes provide a unique environment for plants and animals. A plant may grow in these dunes by using light energy radiated from the sun to transform water and carbon dioxide into chemical energy , which is stored in sugar. When an animal eats the plant, it uses the energy stored in that sugar to heat its body and move around, transforming the chemical energy into kinetic and thermal energy . Though it may not always be obvious, energy transfers and transformations constantly happen all around us and are what enable life as we know it to exist.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
6.1 Electromagnetic Energy
Learning objectives.
- Explain the basic behavior of waves, including travelling waves and standing waves
- Describe the wave nature of light
- Use appropriate equations to calculate related light-wave properties such as frequency, wavelength, and energy
- Distinguish between line and continuous emission spectra
- Describe the particle nature of light
The nature of light has been a subject of inquiry since antiquity. In the seventeenth century, Isaac Newton performed experiments with lenses and prisms and was able to demonstrate that white light consists of the individual colors of the rainbow combined together. Newton explained his optics findings in terms of a "corpuscular" view of light, in which light was composed of streams of extremely tiny particles travelling at high speeds according to Newton's laws of motion. Others in the seventeenth century, such as Christiaan Huygens , had shown that optical phenomena such as reflection and refraction could be equally well explained in terms of light as waves travelling at high speed through a medium called "luminiferous aether" that was thought to permeate all space. Early in the nineteenth century, Thomas Young demonstrated that light passing through narrow, closely spaced slits produced interference patterns that could not be explained in terms of Newtonian particles but could be easily explained in terms of waves. Later in the nineteenth century, after James Clerk Maxwell developed his theory of electromagnetic radiation and showed that light was the visible part of a vast spectrum of electromagnetic waves, the particle view of light became thoroughly discredited. By the end of the nineteenth century, scientists viewed the physical universe as roughly comprising two separate domains: matter composed of particles moving according to Newton's laws of motion, and electromagnetic radiation consisting of waves governed by Maxwell's equations. Today, these domains are referred to as classical mechanics and classical electrodynamics (or classical electromagnetism). Although there were a few physical phenomena that could not be explained within this framework, scientists at that time were so confident of the overall soundness of this framework that they viewed these aberrations as puzzling paradoxes that would ultimately be resolved somehow within this framework. As we shall see, these paradoxes led to a contemporary framework that intimately connects particles and waves at a fundamental level called wave-particle duality, which has superseded the classical view.
Visible light and other forms of electromagnetic radiation play important roles in chemistry, since they can be used to infer the energies of electrons within atoms and molecules. Much of modern technology is based on electromagnetic radiation. For example, radio waves from a mobile phone, X-rays used by dentists, the energy used to cook food in your microwave, the radiant heat from red-hot objects, and the light from your television screen are forms of electromagnetic radiation that all exhibit wavelike behavior.
A wave is an oscillation or periodic movement that can transport energy from one point in space to another. Common examples of waves are all around us. Shaking the end of a rope transfers energy from your hand to the other end of the rope, dropping a pebble into a pond causes waves to ripple outward along the water's surface, and the expansion of air that accompanies a lightning strike generates sound waves (thunder) that can travel outward for several miles. In each of these cases, kinetic energy is transferred through matter (the rope, water, or air) while the matter remains essentially in place. An insightful example of a wave occurs in sports stadiums when fans in a narrow region of seats rise simultaneously and stand with their arms raised up for a few seconds before sitting down again while the fans in neighboring sections likewise stand up and sit down in sequence. While this wave can quickly encircle a large stadium in a few seconds, none of the fans actually travel with the wave-they all stay in or above their seats.
Waves need not be restricted to travel through matter. As Maxwell showed, electromagnetic waves consist of an electric field oscillating in step with a perpendicular magnetic field, both of which are perpendicular to the direction of travel. These waves can travel through a vacuum at a constant speed of 2.998 × × 10 8 m/s, the speed of light (denoted by c ).
All waves, including forms of electromagnetic radiation, are characterized by, a wavelength (denoted by λ , the lowercase Greek letter lambda), a frequency (denoted by ν , the lowercase Greek letter nu), and an amplitude . As can be seen in Figure 6.2 , the wavelength is the distance between two consecutive peaks or troughs in a wave (measured in meters in the SI system). Electromagnetic waves have wavelengths that fall within an enormous range-wavelengths of kilometers (10 3 m) to picometers (10 −12 m) have been observed. The frequency is the number of wave cycles that pass a specified point in space in a specified amount of time (in the SI system, this is measured in seconds). A cycle corresponds to one complete wavelength. The unit for frequency, expressed as cycles per second [s −1 ], is the hertz (Hz) . Common multiples of this unit are megahertz, (1 MHz = 1 × × 10 6 Hz) and gigahertz (1 GHz = 1 × × 10 9 Hz). The amplitude corresponds to the magnitude of the wave's displacement and so, in Figure 6.2 , this corresponds to one-half the height between the peaks and troughs. The amplitude is related to the intensity of the wave, which for light is the brightness, and for sound is the loudness.
The product of a wave's wavelength ( λ ) and its frequency ( ν ), λν , is the speed of the wave. Thus, for electromagnetic radiation in a vacuum:
Wavelength and frequency are inversely proportional: As the wavelength increases, the frequency decreases. The inverse proportionality is illustrated in Figure 6.3 . This figure also shows the electromagnetic spectrum , the range of all types of electromagnetic radiation. Each of the various colors of visible light has specific frequencies and wavelengths associated with them, and you can see that visible light makes up only a small portion of the electromagnetic spectrum. Because the technologies developed to work in various parts of the electromagnetic spectrum are different, for reasons of convenience and historical legacies, different units are typically used for different parts of the spectrum. For example, radio waves are usually specified as frequencies (typically in units of MHz), while the visible region is usually specified in wavelengths (typically in units of nm or angstroms).
Example 6.1
Determining the frequency and wavelength of radiation.
Since c is expressed in meters per second, we must also convert 589 nm to meters.
Check Your Learning
0.353 m = 35.3 cm
Chemistry in Everyday Life
Wireless communication.
Many valuable technologies operate in the radio (3 kHz-300 GHz) frequency region of the electromagnetic spectrum. At the low frequency (low energy, long wavelength) end of this region are AM (amplitude modulation) radio signals (540-2830 kHz) that can travel long distances. FM (frequency modulation) radio signals are used at higher frequencies (87.5-108.0 MHz). In AM radio, the information is transmitted by varying the amplitude of the wave ( Figure 6.5 ). In FM radio, by contrast, the amplitude is constant and the instantaneous frequency varies.
Other technologies also operate in the radio-wave portion of the electromagnetic spectrum. For example, 4G cellular telephone signals are approximately 880 MHz, while Global Positioning System (GPS) signals operate at 1.228 and 1.575 GHz, local area wireless technology (Wi-Fi) networks operate at 2.4 to 5 GHz, and highway toll sensors operate at 5.8 GHz. The frequencies associated with these applications are convenient because such waves tend not to be absorbed much by common building materials.
One particularly characteristic phenomenon of waves results when two or more waves come into contact: They interfere with each other. Figure 6.6 shows the interference patterns that arise when light passes through narrow slits closely spaced about a wavelength apart. The fringe patterns produced depend on the wavelength, with the fringes being more closely spaced for shorter wavelength light passing through a given set of slits. When the light passes through the two slits, each slit effectively acts as a new source, resulting in two closely spaced waves coming into contact at the detector (the camera in this case). The dark regions in Figure 6.6 correspond to regions where the peaks for the wave from one slit happen to coincide with the troughs for the wave from the other slit (destructive interference), while the brightest regions correspond to the regions where the peaks for the two waves (or their two troughs) happen to coincide (constructive interference). Likewise, when two stones are tossed close together into a pond, interference patterns are visible in the interactions between the waves produced by the stones. Such interference patterns cannot be explained by particles moving according to the laws of classical mechanics.
Portrait of a Chemist
Dorothy hodgkin.
Because the wavelengths of X-rays (10-10,000 picometers [pm]) are comparable to the size of atoms, X-rays can be used to determine the structure of molecules. When a beam of X-rays is passed through molecules packed together in a crystal, the X-rays collide with the electrons and scatter. Constructive and destructive interference of these scattered X-rays creates a specific diffraction pattern. Calculating backward from this pattern, the positions of each of the atoms in the molecule can be determined very precisely. One of the pioneers who helped create this technology was Dorothy Crowfoot Hodgkin.
She was born in Cairo, Egypt, in 1910, where her British parents were studying archeology. Even as a young girl, she was fascinated with minerals and crystals. When she was a student at Oxford University, she began researching how X-ray crystallography could be used to determine the structure of biomolecules. She invented new techniques that allowed her and her students to determine the structures of vitamin B 12 , penicillin, and many other important molecules. Diabetes, a disease that affects 382 million people worldwide, involves the hormone insulin. Hodgkin began studying the structure of insulin in 1934, but it required several decades of advances in the field before she finally reported the structure in 1969. Understanding the structure has led to better understanding of the disease and treatment options.
Not all waves are travelling waves. Standing waves (also known as stationary waves ) remain constrained within some region of space. As we shall see, standing waves play an important role in our understanding of the electronic structure of atoms and molecules. The simplest example of a standing wave is a one-dimensional wave associated with a vibrating string that is held fixed at its two end points. Figure 6.7 shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is considered to be one dimensional, since it lies along the length of the string. The motion of string segments in a direction perpendicular to the string length generates the waves and so the amplitude of the waves is visible as the maximum displacement of the curves seen in Figure 6.7 . The key observation from the figure is that only those waves having an integer number, n, of half-wavelengths between the end points can form . A system with fixed end points such as this restricts the number and type of the possible waveforms. This is an example of quantization , in which only discrete values from a more general set of continuous values of some property are observed. Another important observation is that the harmonic waves (those waves displaying more than one-half wavelength) all have one or more points between the two end points that are not in motion. These special points are nodes . The energies of the standing waves with a given amplitude in a vibrating string increase with the number of half-wavelengths n . Since the number of nodes is n – 1, the energy can also be said to depend on the number of nodes, generally increasing as the number of nodes increases.
An example of two-dimensional standing waves is shown in Figure 6.8 , which shows the vibrational patterns on a flat surface. Although the vibrational amplitudes cannot be seen like they could in the vibrating string, the nodes have been made visible by sprinkling the drum surface with a powder that collects on the areas of the surface that have minimal displacement. For one-dimensional standing waves, the nodes were points on the line, but for two-dimensional standing waves, the nodes are lines on the surface (for three-dimensional standing waves, the nodes are two-dimensional surfaces within the three-dimensional volume). Because of the circular symmetry of the drum surface, its boundary conditions (the drum surface being tightly constrained to the circumference of the drum) result in two types of nodes: radial nodes that sweep out all angles at constant radii and, thus, are seen as circles about the center, and angular nodes that sweep out all radii at constant angles and, thus, are seen as lines passing through the center. The upper left image in Figure 6.8 shows two radial nodes, while the image in the lower right shows the vibrational pattern associated with three radial nodes and two angular nodes.
Link to Learning
You can watch the formation of various radial nodes here as singer Imogen Heap projects her voice across a kettle drum.
Blackbody Radiation and the Ultraviolet Catastrophe
The last few decades of the nineteenth century witnessed intense research activity in commercializing newly discovered electric lighting. This required obtaining a better understanding of the distributions of light emitted from various sources being considered. Artificial lighting is usually designed to mimic natural sunlight within the limitations of the underlying technology. Such lighting consists of a range of broadly distributed frequencies that form a continuous spectrum . Figure 6.9 shows the wavelength distribution for sunlight. The most intense radiation is in the visible region, with the intensity dropping off rapidly for shorter wavelength ultraviolet (UV) light, and more slowly for longer wavelength infrared (IR) light.
In Figure 6.9 , the solar distribution is compared to a representative distribution, called a blackbody spectrum, that corresponds to a temperature of 5250 °C. The blackbody spectrum matches the solar spectrum quite well. A blackbody is a convenient, ideal emitter that approximates the behavior of many materials when heated. It is “ideal” in the same sense that an ideal gas is a convenient, simple representation of real gases that works well, provided that the pressure is not too high nor the temperature too low. A good approximation of a blackbody that can be used to observe blackbody radiation is a metal oven that can be heated to very high temperatures. The oven has a small hole allowing for the light being emitted within the oven to be observed with a spectrometer so that the wavelengths and their intensities can be measured. Figure 6.10 shows the resulting curves for some representative temperatures. Each distribution depends only on a single parameter: the temperature. The maxima in the blackbody curves, λ max , shift to shorter wavelengths as the temperature increases, reflecting the observation that metals being heated to high temperatures begin to glow a darker red that becomes brighter as the temperature increases, eventually becoming white hot at very high temperatures as the intensities of all of the visible wavelengths become appreciable. This common observation was at the heart of the first paradox that showed the fundamental limitations of classical physics that we will examine.
Physicists derived mathematical expressions for the blackbody curves using well-accepted concepts from the theories of classical mechanics and classical electromagnetism. The theoretical expressions as functions of temperature fit the observed experimental blackbody curves well at longer wavelengths, but showed significant discrepancies at shorter wavelengths. Not only did the theoretical curves not show a peak, they absurdly showed the intensity becoming infinitely large as the wavelength became smaller, which would imply that everyday objects at room temperature should be emitting large amounts of UV light. This became known as the “ultraviolet catastrophe” because no one could find any problems with the theoretical treatment that could lead to such unrealistic short-wavelength behavior. Finally, around 1900, Max Planck derived a theoretical expression for blackbody radiation that fit the experimental observations exactly (within experimental error). Planck developed his theoretical treatment by extending the earlier work that had been based on the premise that the atoms composing the oven vibrated at increasing frequencies (or decreasing wavelengths) as the temperature increased, with these vibrations being the source of the emitted electromagnetic radiation. But where the earlier treatments had allowed the vibrating atoms to have any energy values obtained from a continuous set of energies (perfectly reasonable, according to classical physics), Planck found that by restricting the vibrational energies to discrete values for each frequency, he could derive an expression for blackbody radiation that correctly had the intensity dropping rapidly for the short wavelengths in the UV region.
The quantity h is a constant now known as Planck's constant, in his honor. Although Planck was pleased he had resolved the blackbody radiation paradox, he was disturbed that to do so, he needed to assume the vibrating atoms required quantized energies, which he was unable to explain. The value of Planck's constant is very small, 6.626 × × 10 −34 joule seconds (J s), which helps explain why energy quantization had not been observed previously in macroscopic phenomena.
The Photoelectric Effect
The next paradox in the classical theory to be resolved concerned the photoelectric effect ( Figure 6.11 ). It had been observed that electrons could be ejected from the clean surface of a metal when light having a frequency greater than some threshold frequency was shone on it. Surprisingly, the kinetic energy of the ejected electrons did not depend on the brightness of the light, but increased with increasing frequency of the light. Since the electrons in the metal had a certain amount of binding energy keeping them there, the incident light needed to have more energy to free the electrons. According to classical wave theory, a wave's energy depends on its intensity (which depends on its amplitude), not its frequency. One part of these observations was that the number of electrons ejected within in a given time period was seen to increase as the brightness increased. In 1905, Albert Einstein was able to resolve the paradox by incorporating Planck's quantization findings into the discredited particle view of light (Einstein actually won his Nobel prize for this work, and not for his theories of relativity for which he is most famous).
Einstein argued that the quantized energies that Planck had postulated in his treatment of blackbody radiation could be applied to the light in the photoelectric effect so that the light striking the metal surface should not be viewed as a wave, but instead as a stream of particles (later called photons ) whose energy depended on their frequency, according to Planck's formula, E = hν (or, in terms of wavelength using c = νλ , E = h c λ E = h c λ ). Electrons were ejected when hit by photons having sufficient energy (a frequency greater than the threshold). The greater the frequency, the greater the kinetic energy imparted to the escaping electrons by the collisions. Einstein also argued that the light intensity did not depend on the amplitude of the incoming wave, but instead corresponded to the number of photons striking the surface within a given time period. This explains why the number of ejected electrons increased with increasing brightness, since the greater the number of incoming photons, the greater the likelihood that they would collide with some of the electrons.
With Einstein's findings, the nature of light took on a new air of mystery. Although many light phenomena could be explained either in terms of waves or particles, certain phenomena, such as the interference patterns obtained when light passed through a double slit, were completely contrary to a particle view of light, while other phenomena, such as the photoelectric effect, were completely contrary to a wave view of light. Somehow, at a deep fundamental level still not fully understood, light is both wavelike and particle-like. This is known as wave-particle duality .
Example 6.2
Calculating the energy of radiation.
2 × × 10 −24 J
Use this simulation program to experiment with the photoelectric effect to see how intensity, frequency, type of metal, and other factors influence the ejected photons.
Example 6.3
Photoelectric effect.
(a) Increasing the brightness of incoming light increases the kinetic energy of the ejected electrons.
(b) Increasing the wavelength of incoming light increases the kinetic energy of the ejected electrons.
(c) Increasing the brightness of incoming light increases the number of ejected electrons.
(d) Increasing the frequency of incoming light can increase the number of ejected electrons.
(b) False. Increasing the frequency of incoming light increases the kinetic energy of the ejected electrons. Frequency is proportional to energy and inversely proportional to wavelength. Frequencies above the threshold value transfer the excess energy into the kinetic energy of the electrons.
(c) True. Because the number of collisions with photons increases with brighter light, the number of ejected electrons increases.
(d) True with regard to the threshold energy binding the electrons to the metal. Below this threshold, electrons are not emitted and above it they are. Once over the threshold value, further increasing the frequency does not increase the number of ejected electrons
Line Spectra
Another paradox within the classical electromagnetic theory that scientists in the late nineteenth century struggled with concerned the light emitted from atoms and molecules. When solids, liquids, or condensed gases are heated sufficiently, they radiate some of the excess energy as light. Photons produced in this manner have a range of energies, and thereby produce a continuous spectrum in which an unbroken series of wavelengths is present. Most of the light generated from stars (including our sun) is produced in this fashion. You can see all the visible wavelengths of light present in sunlight by using a prism to separate them. As can be seen in Figure 6.9 , sunlight also contains UV light (shorter wavelengths) and IR light (longer wavelengths) that can be detected using instruments but that are invisible to the human eye. Incandescent (glowing) solids such as tungsten filaments in incandescent lights also give off light that contains all wavelengths of visible light. These continuous spectra can often be approximated by blackbody radiation curves at some appropriate temperature, such as those shown in Figure 6.10 .
In contrast to continuous spectra, light can also occur as discrete or line spectra having very narrow line widths interspersed throughout the spectral regions such as those shown in Figure 6.13 . Exciting a gas at low partial pressure using an electrical current, or heating it, will produce line spectra. Fluorescent light bulbs and neon signs operate in this way ( Figure 6.12 ). Each element displays its own characteristic set of lines, as do molecules, although their spectra are generally much more complicated.
Each emission line consists of a single wavelength of light, which implies that the light emitted by a gas consists of a set of discrete energies. For example, when an electric discharge passes through a tube containing hydrogen gas at low pressure, the H 2 molecules are broken apart into separate H atoms and we see a blue-pink color. Passing the light through a prism produces a line spectrum, indicating that this light is composed of photons of four visible wavelengths, as shown in Figure 6.13 .
The origin of discrete spectra in atoms and molecules was extremely puzzling to scientists in the late nineteenth century, since according to classical electromagnetic theory, only continuous spectra should be observed. Even more puzzling, in 1885, Johann Balmer was able to derive an empirical equation that related the four visible wavelengths of light emitted by hydrogen atoms to whole integers. That equation is the following one, in which k is a constant:
Other discrete lines for the hydrogen atom were found in the UV and IR regions. Johannes Rydberg generalized Balmer's work and developed an empirical formula that predicted all of hydrogen's emission lines, not just those restricted to the visible range, where, n 1 and n 2 are integers, n 1 < n 2 , and R ∞ R ∞ is the Rydberg constant (1.097 × × 10 7 m −1 ).
Even in the late nineteenth century, spectroscopy was a very precise science, and so the wavelengths of hydrogen were measured to very high accuracy, which implied that the Rydberg constant could be determined very precisely as well. That such a simple formula as the Rydberg formula could account for such precise measurements seemed astounding at the time, but it was the eventual explanation for emission spectra by Neils Bohr in 1913 that ultimately convinced scientists to abandon classical physics and spurred the development of modern quantum mechanics.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry/pages/1-introduction
- Authors: Paul Flowers, William R. Robinson, PhD, Richard Langley, Klaus Theopold
- Publisher/website: OpenStax
- Book title: Chemistry
- Publication date: Mar 11, 2015
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry/pages/6-1-electromagnetic-energy
© Feb 15, 2022 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
It’s a wonderful world — and universe — out there.
Come explore with us!
Science News Explores
Explainer: how heat moves.
Here are the three processes by which energy can be transferred from one place to another

Heat is being transferred from the hot end of this rod to the cold end via conduction, but the hot end of the rod is also radiating heat via that orange glow.
Dvoinik/iStockphoto
Share this:
- Google Classroom
By Sid Perkins
September 30, 2016 at 6:15 am
Throughout the universe, it’s natural for energy to flow from one place to another. And unless people interfere, thermal energy — or heat — naturally flows in one direction only: from hot toward cold.
Heat moves naturally by any of three means. The processes are known as conduction, convection and radiation. Sometimes more than one may occur at the same time.
First, a little background. All matter is made from atoms — either single ones or those bonded in groups known as molecules. These atoms and molecules are always in motion. If they have the same mass, hot atoms and molecules move, on average, faster than cold ones. Even if atoms are locked in a solid, they still vibrate back and forth around some average position.
In a liquid, atoms and molecules are free to flow from place to place. Within a gas, they are even more free to move and will completely spread out within the volume in which they are trapped.
Some of the most easily understood examples of heat flow occur in your kitchen.
Put a pan on a stovetop and turn on the heat. The metal sitting over the burner will be the first part of the pan to get hot. Atoms in the pan’s bottom will start to vibrate faster as they warm. They also vibrate farther back and forth from their average position. As they bump into their neighbors, they share with that neighbor some of their energy. (Think of this as a very tiny version of a cue ball slamming into other balls during a game of billiards. The target balls, previously sitting still, gain some of the cue ball’s energy and move.)
As a result of collisions with their warmer neighbors, atoms start moving faster. In other words, they are now warming. These atoms, in turn, transfer some of their increased energy to neighbors even farther from the original source of heat. This conduction of heat through a solid metal is how the handle of a pan gets hot even though it may be nowhere near the source of heat.
Convection occurs when a material is free to move, such as a liquid or a gas. Again, consider a pan on the stove. Put water in the pan, then turn on the heat. As the pan gets hot, some of that heat transfers to the molecules of water sitting on the bottom of the pan via conduction. That speeds up the motion of those water molecules — they are warming.

As the water warms, it now begins to expand. That makes it less dense. It rises above denser water, carrying away heat from the bottom of the pan. Cooler water flows down to take its place next to the hot bottom of the pan. As this water warms, it expands and rises, ferrying its newly-gained energy with it. In short order, a circular flow of rising warm water and falling cooler water sets up. This circular pattern of heat transfer is known as convection .
It’s also what largely warms food in an oven. Air that’s warmed by a heating element or gas flames at the top or bottom of the oven carries that heat to the central zone where the food sits.
Air that’s warmed at Earth’s surface expands and rises just like the water in the pan on the stove. Large birds such as frigate birds (and human flyers riding engineless gliders) often ride these thermals — rising blobs of air — to gain altitude without using any energy of their own. In the ocean, convection caused by heating and cooling helps to drive ocean currents. These currents move water around the globe.
The third type of energy transfer is in some ways the most unusual. It can move through materials — or in the absence of them. This is radiation.
Consider visible light, a form of radiation. It passes through some types of glass and plastic. X-rays, another form of radiation, readily pass through flesh but are largely blocked by bone. Radio waves pass through the walls of your home to reach the antenna on your stereo. Infrared radiation, or heat, passes through the air from fireplaces and light bulbs. But unlike conduction and convection, radiation doesn’t require a material to transfer its energy. Light, X-rays, infrared waves and radio waves all travel to Earth from the far reaches of the universe. Those forms of radiation will pass through plenty of empty space along the way.
X-rays, visible light, infrared radiation, radio waves are all different forms of electromagnetic radiation . Each type of radiation falls into a particular band of wavelengths. Those types differ in the amount of energy they have. In general, the longer the wavelength, the lower the frequency of a particular type of radiation and the less energy it will carry.
To complicate things, it’s important to note that more than one form of heat transfer may occur at the same time. A stove’s burner not only heats a pan but also the nearby air and makes it less dense. That carries warmth upward via convection. But the burner also radiates heat as infrared waves, making things nearby warm up. And if you’re using a cast-iron skillet to cook a tasty meal, be sure to grab the handle with a potholder: It’s gonna be hot, thanks to conduction!
More Stories from Science News Explores on Physics
Explainer: what is the solar cycle.

Forests could help detect ‘ghost particles’ from space
Explainer: Sprites, jets, ELVES and other storm-powered lights

Here’s why blueberries aren’t blue — but appear to be

The weird sky glow called STEVE is really confusing scientists

Physics explains what happens when a lawn sprinkler sucks in water


Physics explains why poured water burbles the way it does
Scientists Say: 2-D Material
What is a medium in physics?
A medium is defined as the substance that transfers the energy, or light from one substance to another substance or from one place to another, or from one surface to another. The medium acts as a carrier here. The medium can transfer any form of energy, sound wave, light, and heat.
What is a medium in physics examples?
A medium is the substance through which a wave can propagate. Water is the medium of ocean waves. Air is the medium through which we hear sound waves. The electric and magnetic fields are the medium of light.
What is the simple definition of medium?
(Entry 1 of 2) 1 : something that is in a middle position (as in size) 2 : the thing by which or through which something is done Writing is a medium of communication. 3 : the substance in which something lives or acts the medium of air.
What called medium?
As a noun, medium has a few different distinct meanings including: “a middle state or condition,” “the material or technique with which an artist works,” or “one of the means or channels of general communication, information, or entertainment in society, as newspapers, radio, or television.”
How many mediums are there in physics?
There are three different kind of optical mediums based on the passage of light. They are (a) Transparent medium (b) Translucent medium (c) Opague medium. (a) Transparent medium : The medium that allows light to pass through is called transparent medium. Examples: Air, glass, pure water.
What are 3 examples of mediums?
An example of a medium is a newspaper from the combined media form of newspapers, television, magazines, radio and the Internet. (plural mediums or media, painting) A tool used for painting or drawing. Acrylics, oils, charcoal and gouache are all mediums I used in my painting.
What are 3 different types of mediums in science?
- Data storage medium, a storage container in computing.
- Growth medium (or culture medium), in biotechnology, an object in which microorganisms or cells experience growth.
- Interplanetary medium, in astronomy, material which fills the solar system.
What is a medium in Science waves?
A medium is a substance or material that can carry a wave. The wave medium is not the wave and it does not make the wave; it merely carries or transports the wave from its source to other locations. The particles in a medium become disturbed and pass on this disturbance.
What does it mean to use medium?
In art, “medium” refers to the substance the artist uses to create a piece of artwork. For example, the medium Michelangelo used to create “David”(1501-1504) was marble, Alexander Calder’s stabiles employ painted steel plates, and Marcel Duchamp’s infamous “Fountain” (1917) was made with a porcelain medium.
What is medium for light?
It’s the (at least a bit) transparent matter through which light travels. It can be air, gas, glass, diamond, plastic… Light can also travel through vacuum, but it is not usually considered a medium, in particular after Einstein’s relativity theories.
What is the medium answer?
Answer. Medium is a substance through which any material can travel.
Is water a medium?
Water, the most abundant compound on the surface of the Earth and probably in the universe, is the medium of biology, but is much more than that. Water is the most frequent actor in the chemistry of metabolism.
Is air a medium?
Air is a transparent medium.
Do all waves need a medium?
Do all waves require a medium to travel? Explain. No, electromagnetic waves do not require any medium to propagate. No, mechanical waves do not require any medium to propagate.
Is vacuum a medium?
Electromagnetism. In classical electromagnetism, the vacuum of free space, or sometimes just free space or perfect vacuum, is a standard reference medium for electromagnetic effects.
Why do sound waves need a medium?
Sound needs a medium in which to travel. Sound waves cannot form unless there are molecules to bump into each other to pass the wave form along. Sounds will therefore not travel in space where only a vacuum exists.
What are types of wave mediums?
Mechanical Waves are waves which propagate through a material medium (solid, liquid, or gas) at a wave speed which depends on the elastic and inertial properties of that medium. There are two basic types of wave motion for mechanical waves: longitudinal waves and transverse waves.
What is the plural of medium in physics?
medium in Physics topic medium2 ●○○ AWL noun (plural media /-diə/ or mediums) [countable] 1 a way of communicating information and news to people, such as newspapers, television etc → media Advertising is a powerful medium.
What is the difference between medium and media?
In general, if the topic is communication or the arts, “media” is used. If the subject is art or science, “mediums” is more likely to be correct. If you’re describing something of intermediate size or quality and you need an adjective, choose “medium.”
How do waves move through a medium?
Water waves are formed by vibrations in a liquid and sound waves are formed by vibrations in a gas (air). These mechanical waves travel through a medium by causing the molecules to bump into each other, like falling dominoes transferring energy from one to the next.
What is the medium of a mechanical wave?
The Medium The energy of a mechanical wave can travel only through matter. The matter through which the wave travels is called the medium (plural, media). The medium in the water wave pictured above is water, a liquid. But the medium of a mechanical wave can be any state of matter, even a solid.
Can space be a medium for a wave?
Electromagnetic waves are waves in electromagnetic fields. Hence the name. The only thing that cannot be a medium is nothing but vacuum ( or space ) is not nothing there are virtual particle and minimum energy state in it .
Is a photograph a medium?
Photography, in its concrete form (the photograph) functions as a medium in which something is transmitted to a receiver.
Can you say mediums?
For an intervening substance, the correct plural is media. If you are discussing psychics or spiritualists, the correct plural is mediums. For artistic materials, you can use either media or mediums.
What are the medium and technique?
Medium refers to the materials that are used to create a work of art while technique is a process or method using a medium in creating a work of art.
Privacy Overview
Energy Transfers and Transformations
Energy cannot be created or destroyed, but it can be transferred and transformed. There are a number of different ways energy can be changed, such as when potential energy becomes kinetic energy or when one object moves another object.
Earth Science, Physics
Water Boiling Pot
There are three types of thermal energy transfer: conduction, radiation, and convection. Convection is a cyclical process that only occurs in fluids.
Photograph by Liu Kuanxi

Energy , or the power to do work, cannot be created or destroyed. However, energy can change form. It can also move between objects. A common example of energy moving between objects, called energy transfer , is the transfer of kinetic energy from a moving object to a motionless object. Kinetic energy is the energy of motion. When a bat hits a ball, some of the bat's kinetic energy moves to the ball. However, the energy stays in the same form. Thermal energy , or heat, is related to energy from temperature. When something is heated, its temperature rises. That's because its molecules move faster. Temperature measures the "hotness" or "coldness" of an object. "Heat" refers to thermal energy moving from a hotter system to a cooler one. Thermal energy transfers in three ways: conduction , convection , and radiation . Conduction is when thermal energy moves between molecules that are touching each other. If you place a metal spoon in boiling water, the end not touching the water gets hot. This happens because metal is an excellent conductor . This means heat travels through it easily. Some materials, such as wood, are poor conductors. Heat does not travel through them easily. They are known as insulators . Convection only happens in liquids and gases. When water is boiled on a stove, molecules at the bottom of the pot are closest to the heat. They get thermal energy first. They move faster and spread out. This means there are fewer molecules at the bottom of the pot. These molecules rise. They are replaced at the bottom by cooler, denser water. These steps repeat. They create a current of molecules sinking, heating up, rising, cooling down, and sinking again. The third type of heat transfer is called radiation. It is very important to life on Earth. With radiation, a heat source does not have to touch the object being heated. Radiation can even move heat through the emptiness of space. Nearly all thermal energy on Earth comes from the sun. It travels in the form of energy waves, such as light. Materials on Earth take in these waves. They use them for energy or reflect them into space.
Energy Can Change Form Energy can also change form. This is called transformation. For example, a ball on a hill has stored energy because of its position. This energy is called gravitational potential energy . It is the ability of an object to do work because of its position in a gravitational field. The higher on the hill this ball is, the more gravitational potential energy it has. When it rolls down the hill, that potential energy changes into kinetic energy. The ball's kinetic energy is changed into heat by the opposing force of friction. Friction is the force resisting objects sliding against each other. The ball stops at the bottom of the hill because friction transforms all its kinetic energy into heat. As with energy transfers, the amount of energy stays the same in transformations. Energy on a Sand Dune In nature, energy transfers and transformations happen constantly. Look at sand dunes in a coastal environment. Thermal energy shines from the sun. It heats the land and ocean. However, water heats more slowly than land. The temperature difference creates a convection current. This current appears as wind. This wind has kinetic energy. It transfers kinetic energy to sand by carrying it short distances. If the moving sand hits something, it stops because of the friction created. Its kinetic energy is changed into heat energy. As sand builds up, these impacts can create dunes. Sand dunes provide a special environment. Plants grow there. They use light energy to change water and carbon dioxide into energy. That energy is stored in sugar. When an animal eats the plant, it uses the stored energy to heat its body and move around. This transforms the sugar's energy into kinetic and heat energy. Energy transfers and transformations happen constantly. They allow life to exist.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
Energy and Matter Cycles
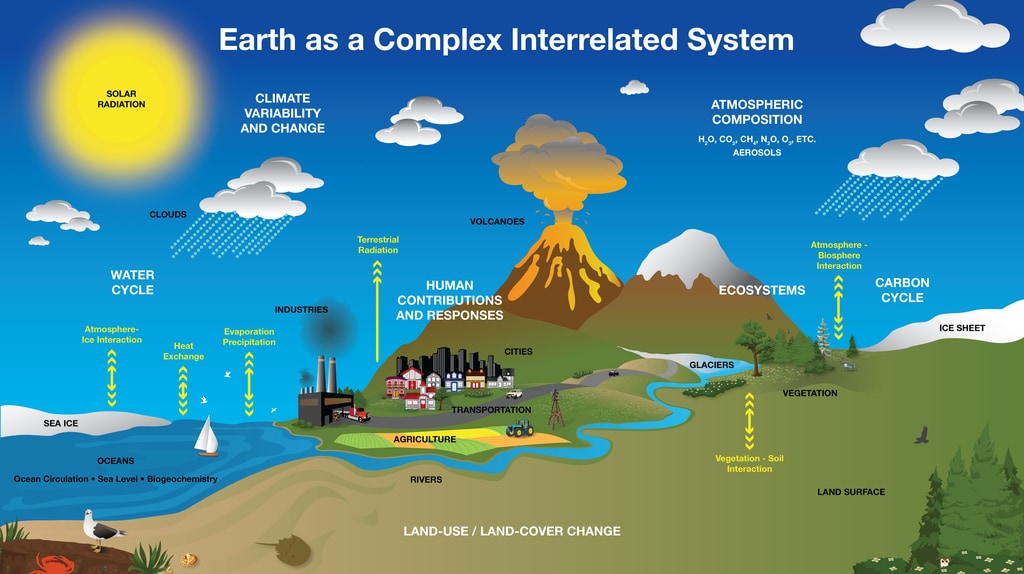
Diagram showing parts of the Earth system. Image Credit: NASA's Goddard Space Flight Center .
Explore the energy and matter cycles found within the Earth System.
Energy Cycle
Energy from the Sun is the driver of many Earth System processes. This energy flows into the Atmosphere and heats this system up It also heats up the Hydrosphere and the land surface of the Geosphere, and fuels many processes in the Biosphere. Differences in the amount of energy absorbed in different places set the Atmosphere and oceans in motion and help determine their overall temperature and chemical structure. These motions, such as wind patterns and ocean currents redistribute energy throughout the environment. Eventually, the energy that began as Sunshine (short-wave radiation) leaves the planet as Earthshine (light reflected by the Atmosphere and surface back into space) and infrared radiation (heat, also called longwave radiation) emitted by all parts of the planet which reaches the top of the Atmosphere. This flow of energy from the Sun, through the environment, and back into space is a major connection in the Earth system; it defines Earth’s climate.
Biogeochemical Cycles:
There are many ways in which the energy, water, and biogeochemical cycles (cycles of the elements that involve life, chemicals, and the solid Earth) interact and influence the Earth System.
Water Cycle (Hydrologic Cycle)
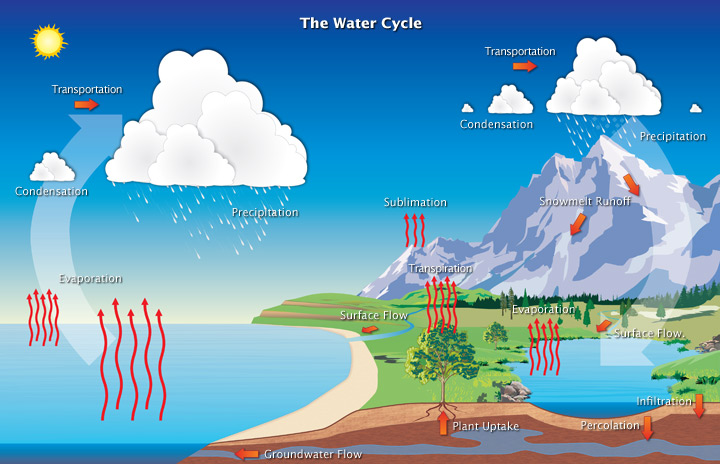
Water is practically everywhere on Earth. Viewed from space, one of the most striking features of our home planet is the water, in both liquid and frozen forms, that covers approximately 75% of the Earth’s surface. Geologic evidence suggests that large amounts of water have likely flowed on Earth for the past 3.8 billion years—most of its existence. Believed to have initially arrived on Earth’s surface through the emissions of ancient volcanoes, water is a vital substance that sets the Earth apart from the rest of the planets in our solar system. In particular, water appears to be a necessary ingredient for the development and nourishment of life.
Water is the only common substance that can exist naturally as a gas, liquid, or solid at the relatively small range of temperatures and pressures found on the Earth’s surface. Sometimes, all three states are even present in the same time and place, such as this wintertime eruption of a geyser in Yellowstone National Park.
In all, the Earth’s water content is about 1.39 billion cubic kilometers (331 million cubic miles), with the bulk of it, about 96.5%, being in the global oceans. As for the rest, approximately 1.7% is stored in the polar icecaps, glaciers, and permanent snow, and another 1.7% is stored in groundwater, lakes, rivers, streams, and soil. Only a thousandth of 1% of the water on Earth exists as water vapor in the atmosphere.
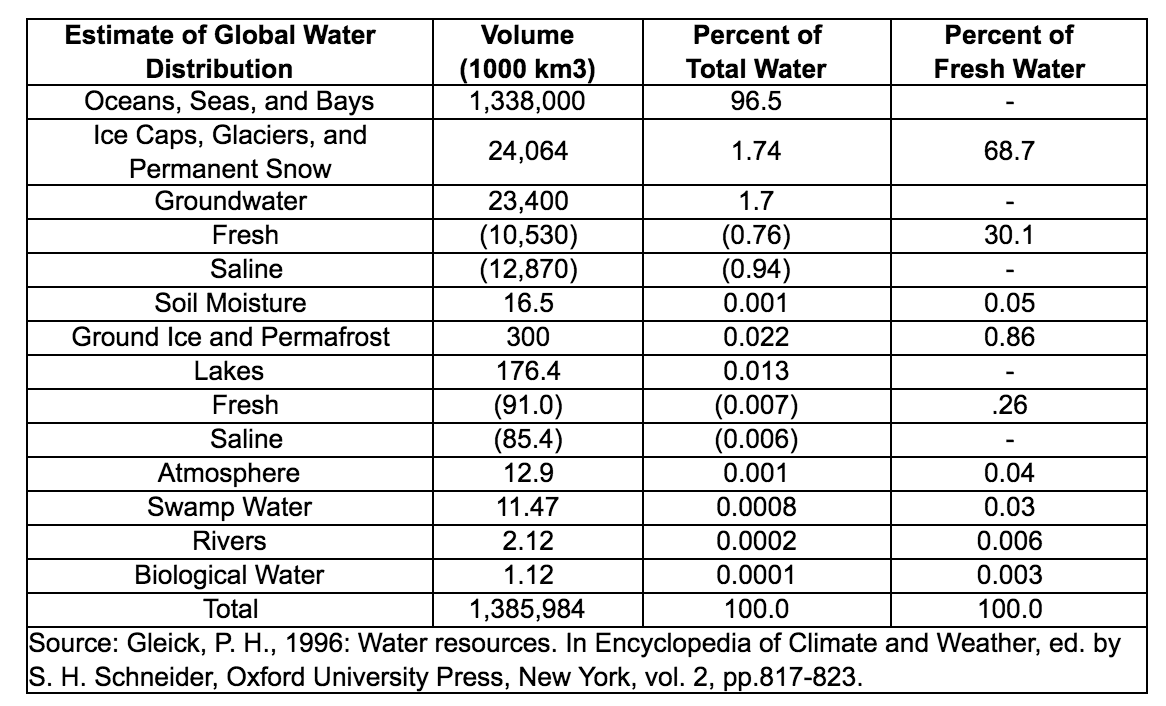
For human needs, the amount of freshwater on Earth—for drinking and agriculture—is particularly important. Freshwater exists in lakes, rivers, groundwater, and frozen as snow and ice. Estimates of groundwater are particularly difficult to make, and they vary widely. (The value in the above table is near the high end of the range.) Groundwater may constitute anywhere from approximately 22 to 30% of fresh water, with ice (including ice caps, glaciers, permanent snow, ground ice, and permafrost) accounting for most of the remaining 78 to 70%.
Groundwater is found in two broadly defined layers of the soil, the “zone of aeration,” where gaps in the soil are filled with both air and water, and, further down, the “zone of saturation,” where the gaps are completely filled with water. The boundary between these two zones is known as the water table, which rises or falls as the amount of groundwater changes.
The amount of water in the atmosphere at any moment in time is only 12,900 cubic kilometers, a minute fraction of Earth’s total water supply: if it were to completely rain out, atmospheric moisture would cover the Earth’s surface to a depth of only 2.5 centimeters. However, far more water—in fact, some 495,000 cubic kilometers of it—are cycled through the Atmosphere every year. It is as if the entire amount of water in the air were removed and replenished nearly 40 times a year.
Despite its small amount, this water vapor has a huge influence on the planet. Water vapor is a powerful greenhouse gas, and it is a major driver of the Earth’s weather and climate as it travels around the globe, transporting latent heat with it. Latent heat is heat obtained by water molecules as they transition from liquid or solid to vapor; the heat is released when the molecules condense from vapor back to liquid or solid form, creating cloud droplets and various forms of precipitation.
Water vapor—and with it energy—is carried around the globe by weather systems. This satellite image shows the distribution of water vapor over Africa and the Atlantic Ocean. White areas have high concentrations of water vapor, while dark regions are relatively dry. The brightest white areas are towering thunderclouds.
The water, or hydrologic, cycle describes the journey of water as water molecules make their way from the Earth’s surface to the Atmosphere and back again, in some cases to below the surface. This gigantic system, powered by energy from the Sun, is a continuous exchange of moisture between the oceans, the atmosphere, and the land.
Water molecules can take an immense variety of routes and branching trails that lead them again and again through the three phases of ice, liquid water, and water vapor. For instance, the water molecules that once fell 100 years ago as rain on your great- grandparents’ farmhouse in Iowa might now be falling as snow on your driveway in California. Water at the bottom of Lake Superior may eventually rise into the atmosphere and fall as rain in Massachusetts. Runoff from the Massachusetts rain may drain into the Atlantic Ocean and circulate northeastward toward Iceland, destined to become part of a floe of sea ice, or, after evaporation to the atmosphere and precipitation as snow, part of a glacier.
Water continually evaporates, condenses, and precipitates, and on a global basis, evaporation approximately equals precipitation. Because of this equality, the total amount of water vapor in the atmosphere remains approximately the same over time. However, over the continents, precipitation routinely exceeds evaporation, and conversely, over the oceans, evaporation exceeds precipitation.
In the case of the oceans, the continual excess of evaporation versus precipitation would eventually leave the oceans empty if they were not being replenished by additional means. Not only are they being replenished, largely through runoff from the land areas, but over the past 100 years, they have been over-replenished: sea level around the globe has risen approximately 17 centimeters over the course of the twentieth century. The main source of this excess runoff from land contributing to sea level rise is the melting of land ice, particularly in Greenland and Antarctica.
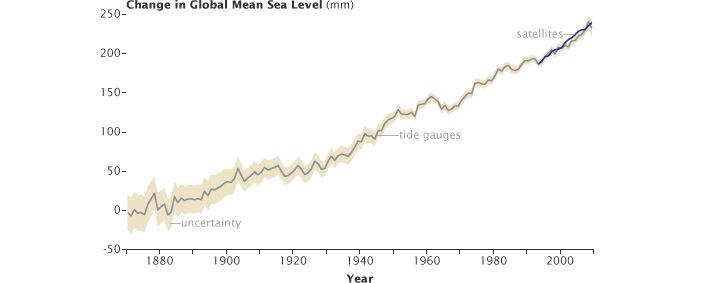
Sea level has risen both because of warming of the oceans, causing water to expand and increase in volume, and because more water has been entering the ocean than the amount leaving it through evaporation or other means. A primary cause for increased mass of water entering the ocean is the calving or melting of land ice (ice sheets and glaciers). Sea ice is already in the ocean, so increases or decreases in the annual amount of sea ice do not significantly affect sea level.

Throughout the hydrologic cycle, there are many paths that a water molecule might follow. Water at the bottom of Lake Superior may eventually rise into the atmosphere and fall as rain in Massachusetts. Runoff from the Massachusetts rain may drain into the Atlantic Ocean and circulate northeastward toward Iceland, destined to become part of a floe of sea ice, or, after evaporation to the atmosphere and precipitation as snow, part of a glacier.
Water molecules can take an immense variety of routes and branching trails that lead them again and again through the three phases of ice, liquid water, and water vapor. For instance, the water molecules that once fell 100 years ago as rain on your great- grandparents’ farmhouse in Iowa might now be falling as snow on your driveway in California.
Evaporation, Transpiration, Sublimation
Together, evaporation, transpiration, and sublimation, plus volcanic emissions, account for almost all the water vapor in the Atmosphere that isn’t inserted through human activities. Studies show that evaporation—the process by which water changes from a liquid to a gas—from oceans, seas, and other bodies of water (lakes, rivers, streams) provides nearly 90% of the moisture in our atmosphere. Most of the remaining 10% found in the atmosphere is released by plants through transpiration where plants take in water through their roots, then release it through small pores on the underside of their leaves. For example, a cornfield 1 acre in size can transpire as much as 4,000 gallons of water every day. In addition, a very small portion of water vapor enters the Atmosphere through sublimation, the process by which water changes directly from a solid (ice or snow) to a gas. The gradual shrinking of snow banks in cases when the temperature remains below freezing results from sublimation.
Condensation & Precipitation
After the water enters the lower atmosphere, rising air currents carry it upward, often high into the atmosphere, where the rising air cools. In cooled air, water vapor is more likely to condense from a gas to a liquid to form cloud droplets. Cloud droplets can grow and produce precipitation (including rain, snow, sleet, freezing rain, and hail), which is the primary mechanism for transporting water from the atmosphere back to the Earth’s surface.
When precipitation falls over the land surface, it follows various routes in its subsequent paths. Some of it evaporates, returning to the atmosphere; some seeps into the ground as soil moisture or groundwater; and some runs off into rivers and streams. Almost all of the water eventually flows into the oceans or other bodies of water, where the cycle continues. At different stages of the cycle, some of the water is intercepted by humans or other life forms for drinking, washing, irrigating, and a large variety of other uses.
Sea Level Rise
Sea level has been rising over the past century, partly due to thermal expansion of the ocean as it warms, causing water to expand and increase in volume, and partly due to the melting of glaciers and ice caps because more water has been entering the ocean than the amount leaving it through evaporation or other means. (Graph ©2010 Australian Commonwealth Scientific and Research Organization.)
Snow and Ice Melt
A primary cause for increased mass of water entering the ocean is the calving or melting of land ice (ice sheets and glaciers). Sea ice is already in the ocean, so increases or decreases in the annual amount of sea ice do not significantly affect sea level.
Credit: NASA Earth Observatory
The Changing Nitrogen Cycle
Credit: UCAR Center for Science Education
Plants and animals could not live without the essential element, nitrogen. It makes up many biological structures and processes such as cells, amino acids, proteins, and even DNA. It is also necessary for plants to produce chlorophyll, which they use in photosynthesis to make their food and energy.
Nitrogen forms simple chemicals called amino acids, the essential building blocks of all proteins, enzymes, and especially DNA. It helps plants use carbohydrates to gain energy, like certain foods we eat help us to gain energy. Nitrogen controls how plants take their form and how they function inside, and nitrogen helps plants make the protein that helps them grow strong and healthy. Humans and animals benefit from eating vegetables and plants that are rich in nitrogen because proteins are passed on to humans and animals when they eat vegetables and plants.
We might commonly think of Earth as having an oxygen-dominated atmosphere, but in reality, the molecule makes up a little less than 20% our air. Most of what surrounds us is nitrogen, at 78 percent in the form of diatomic nitrogen gas, the gas itself is very unreactive. plants and animals simply cannot absorb the gas directly from the atmosphere. Nitrogen, in the forms of Nitrates (NO3), Nitrites (NO2), and Ammonium (NH4), is a nutrient needed for plant growth. Plants take up nitrogen in forms of nitrate ( NO3-) and ammonium ( NH4+ ). Most plants thrive on equal amounts of these ions but nitrates are more quickly available to plants because they move through the soil solution, whereas ammonium ions become fixed or held on to clay particles, called colloids, because of their positive charge.
How Plants Take Up Nitrogen
The nitrogen cycle involves certain processes that change nitrogen into different forms. Unfortunately, these forms of nitrogen are not always used by plants because they either get onto clay particles in soil, they leach into the groundwater because they cannot be absorbed by the soil, or they change into nitrogen gases that escape into Earth's atmosphere. So how does nitrogen change states from N2 in the air to these other states so that they are accessible by the Biosphere? Luckily there are specific kinds of microorganisms living in the soil that can convert gaseous forms of nitrogen into inorganic nitrogen that plants can use.
Specialized bacteria in soil (and certain types of algae in water) can fix nitrogen. These bacteria that cling to roots within the soil convert (or "fix") this inorganic nitrogen into organic forms (ammonia and nitrate ions) that plants can absorb. This process of converting nitrogen to a “biologically available” form - in other words, converting nitrogen gas to a form that plants can use - is referred to as nitrogen fixation. Lightning strikes also result in some nitrogen fixation by splitting the nitrogen molecule into free nitrogen, which immediately reacts with oxygen in the air to form nitrogen oxides. Some of these nitrogen oxide gases dissolve in rainwater and eventually percolate into the soil (Pedosphere). The nutrients needed for plant growth are drawn from the soil from the roots to the leaves. Therefore, any organism (including humans) consuming the nuts, leaves, seeds, roots, tubercles, or fruits of plants can digest this organic form of nitrogen. The Nitrogen Cycle is this process of moving nitrogen among plants, animals, bacteria, the atmosphere, and soil in the ground. This cycle is continuous.
Human activities have a large impact on global nitrogen cycles. In agriculture, soils are generally not rich enough in fixed nitrogen to sustain repetitive crop yields year after year; as a result, farmers use compost heaps or add industrially mass-produced fertilizers such as ammonium nitrate (containing high amounts of organic nitrogen), to enhance the soil.
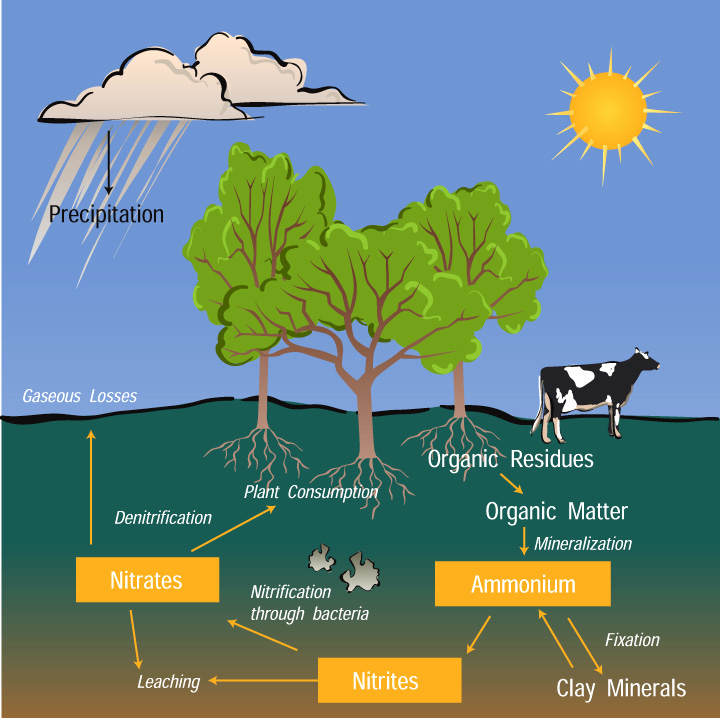
What Happens When Plants Don't Get Enough Nitrogen:
Plants deficient in nitrogen have thin, spindly stems and their growth is stunted. Their older leaves turn yellowish-green from the lack of chlorophyll produced in the leaves (chlorosis), while newer leaves are supplied with the available nitrogen and sufficient chlorophyll.
What Happens When Plants Get Too Much Nitrogen:
Plants that get too much nitrogen have a lot of foliage (leaf) growth but are not strong. Plants that are not strong can get diseases more easily, can be bothered more by bugs, and can eventually fall over and die. An excess amount of nitrogen in plants can affect the amount of sugar and vitamins in fruits and vegetables, making them taste different. More importantly, excess nitrogen can build up in plant tissues causing toxicity (poisoning) in livestock and in small children who eat nitrogen-rich, leafy vegetables. As we produce synthetic fertilizers, burn fossil fuels, grow legumes such as soybeans as a crop (which fix nitrogen), and clear, burn, and drain wetlands, we release nitrogen in forms that plants use. We have made the amount of biologically available nitrogen through human activity much greater than the nitrogen fixed by bacteria, algae, and lightning.
The Nitrogen Cycle Processes:
- Fixation - Fixation is the first step in the process of making nitrogen usable by plants. Here bacteria change nitrogen into ammonium.
- Nitrification - This is the process by which ammonium gets changed into nitrates by bacteria. Nitrates are what the plants can then absorb.
- Assimilation - This is how plants get nitrogen. They absorb nitrates from the soil into their roots. Then the nitrogen gets used in amino acids, nucleic acids, and chlorophyll.
- Ammonification (or mineralization) - This is part of the decaying process. When a plant or animal dies, decomposers like fungi and bacteria turn the nitrogen back into ammonium so it can reenter the nitrogen cycle.
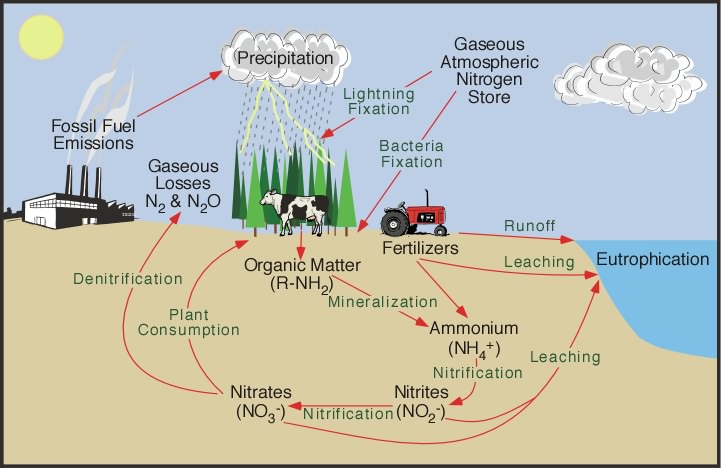
Nitrogen As a Pollutant in the Atmosphere
Nitrogen dioxide (NO2) is a gas that occurs naturally in our atmosphere, but in concentrations very low as compared to oxygen (O2) and nitrogen (N2). NO2 is also common pollutant produced primarily during the combustion of gasoline in vehicle engines and coal in power plants. NO2 is part of a family of chemical compounds collectively called “nitrogen oxides” or “NOx”. Nitric oxide (NO) is also part of the NOx family. Together, NO and NO2 play important roles in the chemical formation of ozone near the Earth's surface, as well as contribute to smog when combined with oxygen molecules and the fumes from paint and gasoline (called Volatile Organic Compounds or VOC’s). These compounds also contribute to the production of acid rain when mixed with water vapor forming nitric acid.
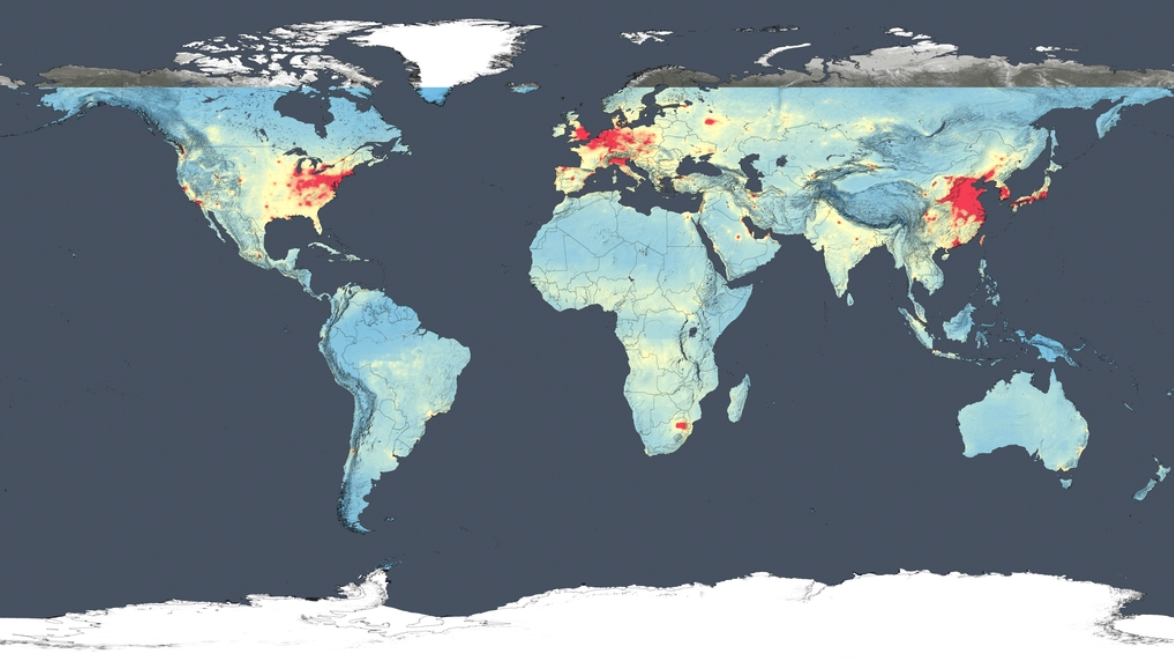
Using new, high-resolution global satellite maps of air quality indicators, NASA scientists tracked air pollution trends over the last decade in various regions and 195 cities around the globe. According to recent NASA research findings, the United States, Europe, and Japan have improved air quality thanks to emission control regulations, while China, India and the Middle East, with their fast-growing economies and expanding industry, have seen more air pollution.
Ozone occurs naturally in the air we breathe, but there's not enough of it to hurt us. Ozone high in the atmosphere (i.e., in the stratospheric “ozone layer”) protects us; it is like sunscreen, protecting us from harmful ultraviolet (UV) rays from the Sun. Near the ground though, ozone is a pollutant. It damages our lungs and harms plants, including the plants we eat. Unhealthy levels of ozone form when there is a lot of NO2 in the air. NO2—and ozone—concentrations are usually highest in cities since NO2 is released into the atmosphere when we burn gas in our cars or coal in our power plants, both things that happen more in cities.
Nitrogen dioxide breaks apart in sunlight releasing free oxygen atoms to connect onto oxygen molecules forming dangerous ground-level ozone. Since sunlight is an important ingredient in the formation of high concentrations of ozone, ozone in urban areas tends to be greatest in summer when sunlight is strongest. NO2 is also unhealthy to breathe in high concentrations, such as on busy streets and highways where there are lots of cars and trucks. When driving, it is typically a good idea to keep the car windows rolled up and the car's ventilation set to “recirculate” so as to keep pollution out of the interior of the car. It is also important to reduce outdoor activities like playing or jogging if government officials warn you that air quality will be bad on a certain day.
Nitrous oxide (N20) is a powerful greenhouse gas, which traps heat near the Earth’s surface. You may have heard of it before, referred to as “laughing gas” which is a common medical treatment to decrease pain. It is produced almost entirely at the Earth's surface, about 70% from through natural processes in the Earth’s Biosphere (tiny microbes that alter nitrogen in the soils of tropical forests and in the oceans) and the rest from human activities (e.g. from farm animals, sewage, and fertilizers, as well as fossil-fuel burning). Quantities of nitrous oxide have increased since the Industrial Revolution in the Atmosphere as Earth’s climate has gotten warmer. scientists have observed an increase in N2O of about 0.3%/year since the 1950's.
For more information about the Nitrogen Cycle, visit UCAR.
The Carbon Cycle

Carbon is a fundamental part of the Earth system. It is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. Forged in the heart of aging stars, carbon is the fourth most abundant element in the Universe. Most of Earth’s carbon—about 65,500 billion metric tons—is stored in rocks. The rest is in the ocean, atmosphere, plants, soil, and fossil fuels. Carbon moves from the atmosphere to the land, ocean, and life through biological, chemical, geological and physical processes in a cycle called the carbon cycle. Any change in the cycle that shifts carbon out of one reservoir puts more carbon in the other reservoirs. Carbon flows between each reservoir in slow and fast cycles.
This diagram of the fast carbon cycle shows the movement of carbon between land, atmosphere, and oceans. Yellow numbers are natural fluxes, and red are human contributions in gigatons of carbon per year. White numbers indicate stored carbon.
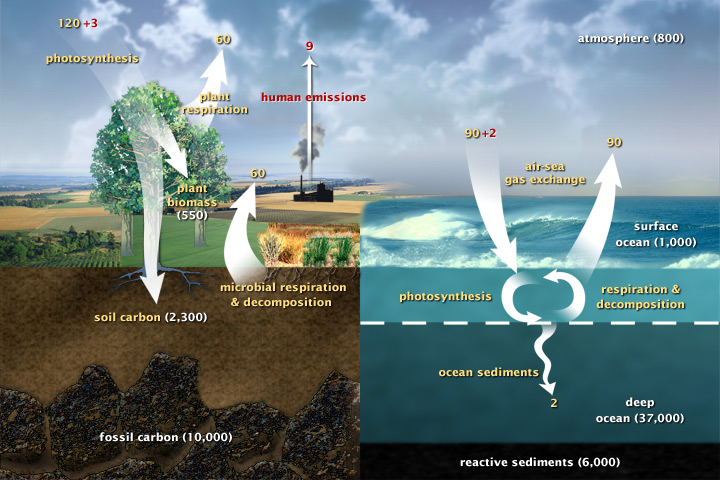
Over the long term, the carbon cycle seems to maintain a balance that prevents all of Earth’s carbon from entering the atmosphere (as is the case on Venus) or from being stored entirely in rocks. This balance helps keep Earth’s temperature relatively stable, like a thermostat. This thermostat works over a few hundred thousand years, as part of the slow carbon cycle. This means that for shorter time periods—tens to a hundred thousand years—the temperature of Earth can vary. And, in fact, Earth swings between ice ages and warmer interglacial periods on these time scales. Parts of the carbon cycle may even amplify these short-term temperature changes.
On very long time scales (millions to tens of millions of years), the movement of tectonic plates and changes in the rate at which carbon seeps from the Earth’s interior may change the temperature on Earth’s thermostat. Earth has undergone such a change over the last 50 million years, from the extremely warm climates of the Cretaceous (roughly 145 to 65 million years ago) to the glacial climates of the Pleistocene (roughly 1.8 million to 11,500 years ago). [See Divisions of Geologic Time—Major Chronostratigraphic and Geochronologic Units for more information about geological eras.]
Slow Cycle
Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 million years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, 1013 to 1014 grams (10–100 million metric tons) of carbon move through the slow carbon cycle every year. In comparison, human emissions of carbon to the atmosphere are on the order of 1015 grams (1 Billion Metric Tons), whereas the fast carbon cycle moves 1016 to 1017 grams (10-100 billion Billion Metric Tons) of carbon per year.
The movement of carbon from the Atmosphere to the Geosphere (rocks) begins with rain. Atmospheric carbon combines with water to form a weak acid—carbonic acid—that falls to the surface in rain. The acid dissolves rocks—a process called chemical weathering—and releases calcium, magnesium, potassium, or sodium ions. Rivers carry the ions to the ocean. Rivers carry calcium ions—the result of chemical weathering of rocks—into the ocean, where they react with carbonate dissolved in the water. The product of that reaction, calcium carbonate, is then deposited onto the ocean floor, where it becomes limestone. In the ocean, the calcium ions combine with bicarbonate ions to form calcium carbonate, the active ingredient in antacids and the chalky white substance that dries on your faucet if you live in an area with hard water. In the modern ocean, most of the calcium carbonate is made by shell-building (calcifying) organisms (such as corals) and plankton (like coccolithophores and foraminifera). After the organisms die, they sink to the seafloor. Over time, layers of shells and sediment are cemented together and turn to rock, storing the carbon in stone—limestone and its derivatives. Limestone, or its metamorphic cousin, marble, is rock made primarily of calcium carbonate. These rock types are often formed from the bodies of marine plants and animals, and their shells and skeletons can be preserved as fossils. Carbon locked up in limestone can be stored for millions—or even hundreds of millions—of years. Only 80 percent of carbon-containing rock is currently made this way. The remaining 20 percent contain carbon from living things (organic carbon) that have been embedded in layers of mud. Heat and pressure compress the mud and carbon over millions of years, forming sedimentary rock such as shale. In special cases, when dead plant matter builds up faster than it can decay, layers of organic carbon become oil, coal, or natural gas instead of sedimentary rock like shale. This coal seam in Scotland was originally a layer of sediment, rich in organic carbon. The sedimentary layer was eventually buried deep underground, and the heat and pressure transformed it into coal. Coal and other fossil fuels are a convenient source of energy, but when they are burned, the stored carbon is released into the atmosphere. This alters the balance of the carbon cycle and is changing Earth’s climate.

The slow cycle returns carbon to the atmosphere through volcanoes. Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. The heated rock recombines into silicate minerals, releasing carbon dioxide. When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year—100–300 times more than volcanoes—by burning fossil fuels.
Chemistry regulates this dance between ocean, land, and atmosphere. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering. Carbon stored in rocks is naturally returned to the atmosphere by volcanoes. In this photograph, Russia’s Kizimen Volcano vents ash and volcanic gases in January 2011. Kizimen is located on the Kamchatka Peninsula, where the Pacific Plate is subducting beneath Asia. However, the slow carbon cycle also contains a slightly faster component: the ocean. At the surface, where air meets water, carbon dioxide gas dissolves in and ventilates out of the ocean in a steady exchange with the atmosphere. Once in the ocean, carbon dioxide gas reacts with water molecules to release hydrogen ions, making the ocean more acidic. The hydrogen reacts with carbonate from rock weathering to produce bicarbonate ions.
Before the industrial age, the ocean vented carbon dioxide to the atmosphere in balance with the carbon the ocean received during rock weathering. However, since carbon concentrations in the atmosphere have increased, the ocean now takes more carbon from the atmosphere than it releases. Over millennia, the ocean will absorb up to 85 percent of the extra carbon people have put into the atmosphere by burning fossil fuels, but the process is slow because it is tied to the movement of water from the ocean’s surface to its depths.In the meantime, winds, currents, and temperature control the rate at which the ocean takes carbon dioxide from the atmosphere. (See The Ocean’s Carbon Balance on the Earth Observatory.) It is likely that changes in ocean temperatures and currents helped remove carbon from and then restore carbon to the atmosphere over the few thousand years in which the ice ages began and ended. This means that for shorter time periods—tens to a hundred thousand years—the temperature of Earth can vary. And, in fact, Earth swings between ice ages and warmer interglacial periods on these time scales. Parts of the carbon cycle may even amplify these short-term temperature changes.The uplift of the Himalaya, beginning 50 million years ago, reset Earth’s thermostat by providing a large source of fresh rock to pull more carbon into the slow carbon cycle through chemical weathering. The resulting drop in temperatures and the formation of ice sheets changed the ratio between heavy and light oxygen in the deep ocean, as shown in this graph. (Graph based on data from Zachos at al., 2001.)

The time it takes carbon to move through the fast carbon cycle is measured in a lifespan, which may not seem very quick. The fast carbon cycle is largely the movement of carbon through life forms on Earth or the Biosphere. Between 1015 and 1017 grams (1,000 to 100,000 million metric tons) of carbon move through the fast carbon cycle every year. On this time scale, the carbon cycle is most visible in life. Carbon plays an essential role in biology because of its ability to form many bonds—up to four per atom—in a seemingly endless variety of complex organic molecules. Plants and phytoplankton (microscopic organisms in the ocean) are the main components of the fast carbon cycle and convert carbon dioxide to biomass (like leaves and stems) through photosynthesis. In this process, they take carbon dioxide (CO2) from the atmosphere by absorbing it into their cells with water to form sugar (CH2O) and oxygen. The chemical reaction looks like this:
CO2 + H2O + energy = CH2O + O2
During photosynthesis, plants absorb carbon dioxide and sunlight to create fuel—glucose and other sugars—for building plant structures. This process forms the foundation of the fast (biological) carbon cycle. (Illustration adapted from P.J. Sellers et al., 1992.)The bonds in the long carbon chains contain a lot of energy. When the chains break apart, the stored energy is released. This energy makes carbon molecules an excellent source of fuel for all living things. The carbon returns to the Atmosphere in the following processes but all involve the same chemical reaction:
- when plants and phytoplankton break down the sugar to get the energy they need to grow
- when plants and phytoplankton die or decay (and are eaten by bacteria) at the end of the growing season
- when plants and phytoplankton are eaten and digested by animals (including people) to get energy
- when plants and phytoplankton burn in fires
In each case, oxygen combines with sugar to release water, carbon dioxide, and energy. The basic chemical reaction looks like this:
CH2O + O2 = CO2 + H2O + energy
These four processes move carbon from a plant and put carbon gases into the Atmosphere. Changes that return carbon to the Atmosphere result in warmer temperatures on Earth.
The fast carbon cycle is so tightly tied to plant life that the growing season can be seen by the way carbon dioxide fluctuates in the atmosphere. In the Northern Hemisphere winter, when few land plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. During the spring, when plants begin growing again, concentrations drop. It is as if the Earth is breathing. Because plants and animals are an integral part of the carbon cycle, the carbon cycle is closely connected to ecosystems. As ecosystems change under a changing climate, the carbon cycle will also change. For example, plants may bloom earlier in the year and grow for more months (assuming sufficient water is present) as the growing season gets longer, altering the food supply for animals in the ecosystem. If more plants grow, they will take more carbon out of the atmosphere and cool temperatures. If, on the other hand, warming slows plant growth, habitats will shift and more carbon will go into the atmosphere where it can cause additional warming.
Related Models
Air quality story map.

Ocean Circulation Patterns: Garbage Patches Story Map
Volcanic Eruptions Story Map

Related Mini Lessons
Energy and matter: water cycle & the ocean's temperature.
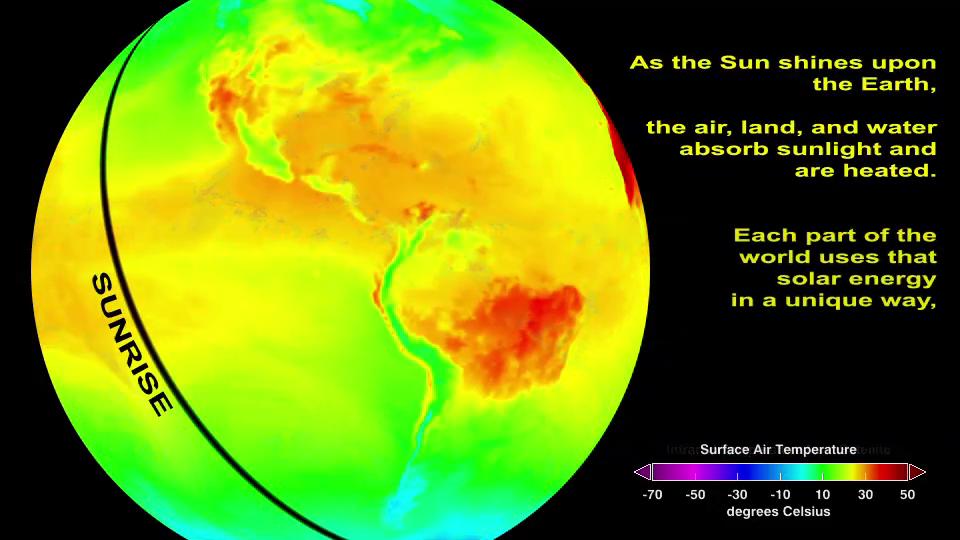
Energy and Matter: Exploring Ocean Salinity
Energy and Matter: Dust Transport
Related Lesson Plans
Observing earth’s seasonal changes.
Earth’s Energy Budget-Seasonal Cycles
Smoke travels.
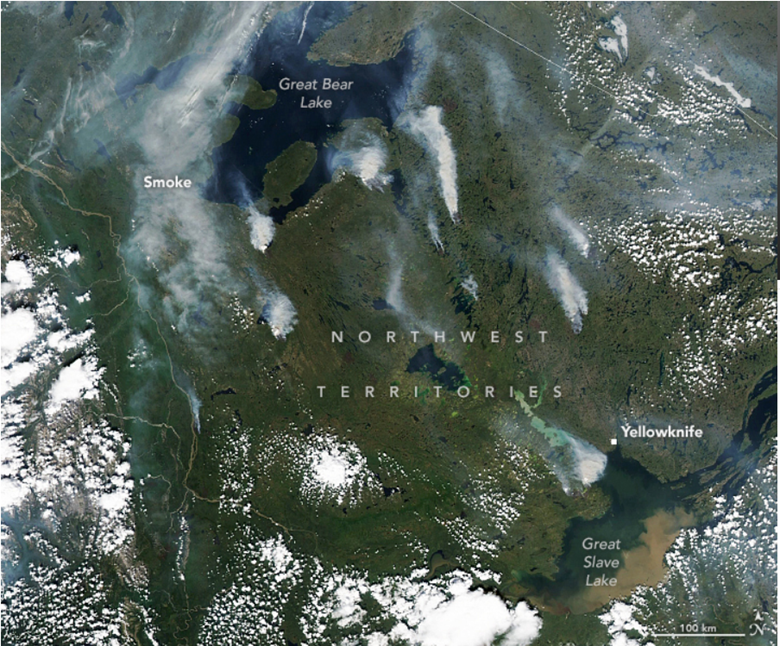
Related Links
- Matter and Energy Cycles Mini Lessons
- Matter and Energy Cycles Interactive Lessons
- Matter and Energy Cycles Lesson Plans
- NASA Earth Observatory
Download this page


- April 8, 2024 | What Came First the Chicken or the Egg? Discovery of Ancient Eggshells Rewrites Chicken History
- April 8, 2024 | Revolutionizing AI With the Power of Energy Efficiency
- April 8, 2024 | Motion Camouflage: The Remarkable Hunting Tactics of Trumpetfish
- April 8, 2024 | Researchers Discover Possible Solutions To Reverse the Impact of Alzheimer’s Disease
- April 8, 2024 | Magnetic Awakening: Unusual Radio Pulses Detected From a Previously Dormant Star
Science Simplified: What Are Dark Matter and Dark Energy?
By Argonne National Laboratory March 30, 2024

Dark matter and dark energy, making up most of the universe, are elusive forces that shape cosmic structure and expansion. Scientists worldwide, including at Argonne National Laboratory, are using advanced technology to unravel these mysteries. Credit: SciTechDaily.com
What Are Dark Matter and Dark Energy?
There’s something amiss in the cosmos. Mysterious influences seem to be stretching the universe apart and clumping stuff together in unexpected ways, but we can’t see or touch them. Scientists call these influences dark energy and dark matter.
Humans have studied the sky for many thousands of years, and in the last century, scientists have really started to understand how the universe moves and changes under the influence of a force called gravity. Gravity affects everything, including not only matter — a scientific term for stuff — but also light. It’s what pulls our bodies into Earth, and it also works over the vast distances between stars and galaxies.
Gravity plays a crucial role in how galaxies form and move around. As scientists learn more about the universe, they find that much of the behavior of galaxies wouldn’t make sense unless there were a huge amount of invisible matter present — way more matter than we have yet to uncover. This invisible — or dark — matter exerts an extra gravitational pull. If it did not exist, some galaxies would fly apart, and some wouldn’t have formed at all.
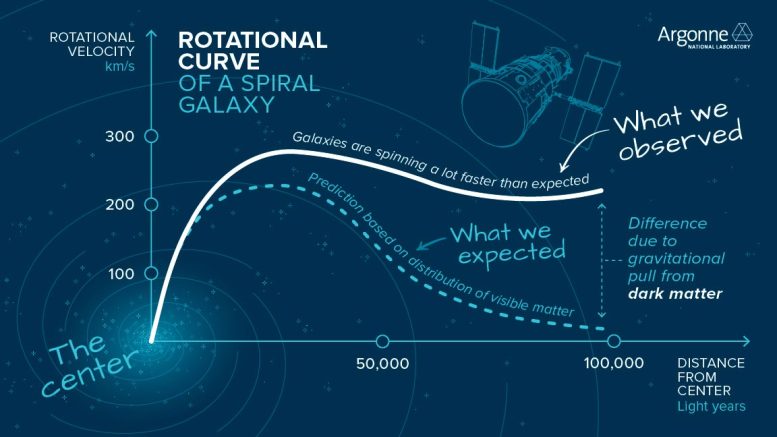
This graph shows a realistic example of how dark matter can cause outer regions of spiral galaxies to rotate faster than they would if they were only under the influence of gravity from visible matter. This discrepancy suggests that dark matter exists, exerting an additional gravitational pull. Credit: Argonne National Laboratory
We call it “dark” because we can’t see it. Unlike visible matter (matter we can see, including stars, planets, water, etc.), it doesn’t appear to release or absorb light, or interact with other matter except through gravity. We know where it should be, but nothing is there when we look. It’s like seeing ripples in a pond, but not being able to see what caused them.
Meanwhile, something else is driving the universe to expand faster and faster. The universe, as far as we can tell, has been expanding since it began 13.8 billion years ago. The space between objects is ever-increasing, as if space itself is being stretched apart like the surface of a balloon as it’s inflated. Scientists expected that the speed of this expansion would slow down with time, but instead, they’ve discovered the opposite. Around five billion years ago, the universe’s expansion started to speed up. We don’t know what’s causing this accelerated expansion, but we named it dark energy.
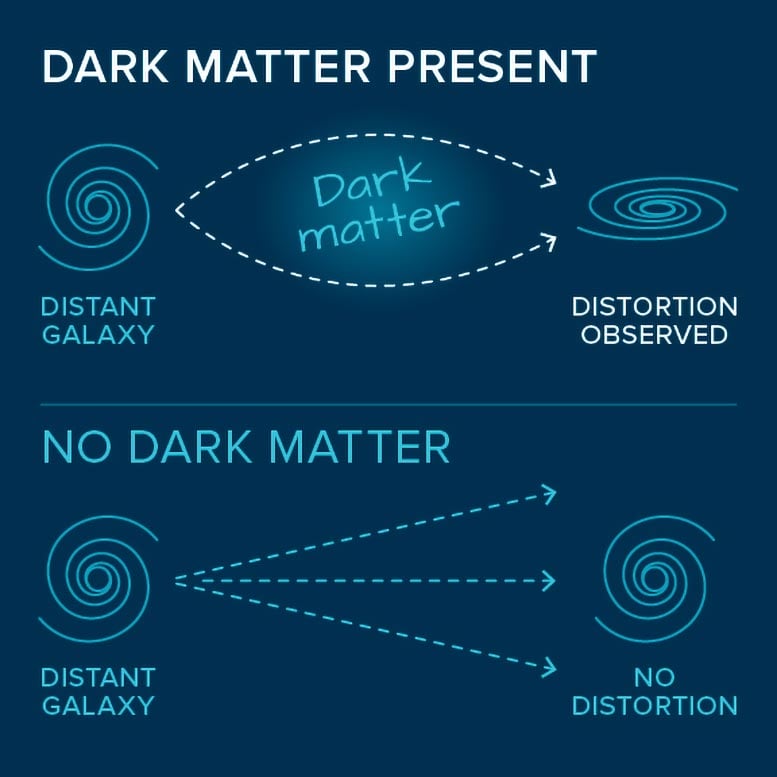
Gravitational pull from dark matter can bend light traveling from distant galaxies, causing their images to appear distorted when they reach our telescopes. This phenomenon, called gravitational lensing, reveals dark matter’s presence even though we can’t see it. Credit: Argonne National Laboratory
From what scientists can tell, visible matter makes up only 5% of the universe. Dark matter and dark energy are believed to make up the other 27% and 68%, respectively. In other words, what we know well — visible matter — doesn’t even come close to explaining the nature of the vast majority of the universe.
So, how are scientists trying to solve this mystery? What are dark matter and dark energy?
In order to find out, we need data, and lots of it. To collect this data, scientists build giant telescopes and cameras. These include the Hubble and James Webb Space Telescopes in outer space; the South Pole Telescope in Antarctica; the Dark Energy Spectroscopic Instrument in Arizona; and the Dark Energy Survey and forthcoming Vera C. Rubin Observatory in Chile.
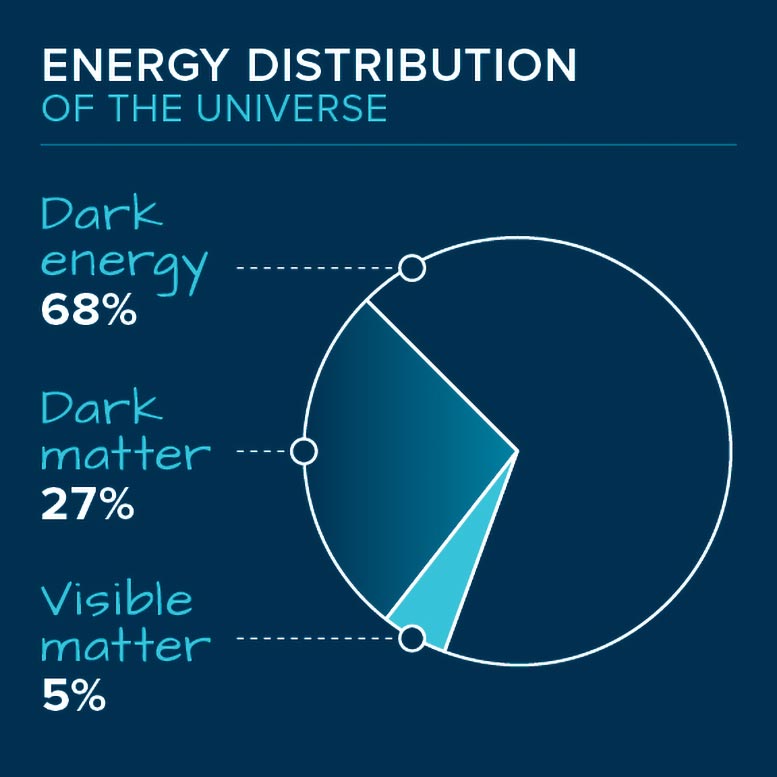
The universe is predominantly composed of dark energy and dark matter. Visible matter (everything we can see, including stars and planets) only makes up around 5% of the universe. Scientists are investigating the nature of the unknown 95%. Credit: Argonne National Laboratory
These sensitive instruments survey the sky to reveal the location and movement of galaxies in the universe over time. Supercomputers help scientists run detailed simulations of the universe as well as analyze the data from the telescopes. In addition to looking at the sky for answers, scientists are also building sensitive detectors to directly search for dark matter on Earth.
Researchers at the U.S. Department of Energy’s Argonne National Laboratory contribute to the study of dark matter and dark energy through participation in these large cosmological surveys, particle physics experiments and by using advanced computing and simulation. Information from these surveys and simulations helps scientists to create maps of where dark matter exists and provides clues about the nature of dark energy
As our telescopes, supercomputers, and other instruments get more sophisticated, we find more and more evidence that we are missing something big, and scientists are working to understand what it might be. The work of Argonne scientists is bringing the world closer to unraveling these cosmic mysteries.
More on SciTechDaily

Gamma-Ray Bursts: Unraveling the Mysteries of the Universe With the Most Powerful Explosions

Peeking at Schrodinger’s Cat Without Disturbing It – A Way of Measuring a Quantum System While Keeping Its Superposition Intact
Parkinson’s, Cancer, and Type 2 Diabetes Share a Key Element That Drives Disease

Extraordinary Species Diversity Within a 14.7 Million-Year-Old Tropical Rainforest and Sheds Light on Evolution

Spin-Based Quantum Computing Breakthrough: Physicists Achieve Tunable Spin Wave Excitation

Inaccuracies in Genetic Studies Exposed by NIH – European Ancestry Under the Microscope

Jupiter’s Moon Europa Could Be Pulling Oxygen Down Below the Ice To Feed Life

“Invisible” Gold – Scientists Discover “Fool’s Gold” Is Not So Foolish After All
8 comments on "science simplified: what are dark matter and dark energy".
You’ve got it all wrong, especially with dark energy. Both special and general relativity show us that time slows and space “shrinks”. If time goes faster then space gets bigger. This is all in the math’ of the theory. Why don’t you take time as fundamental and space as merely the effect of time passing? It’s quite simple and logical if you think about it. That way, you get quickening time and cosmic expansion. You know, just like we observe right? It is naive to invent some mysterious energy that drives expansion when the answer is staring us in the face. Red shift has two causes you know. One is the Doppler effect but there is another we are neglecting – the quickening of time. Look into the past and all the clocks went slower, giving us a red shift of everything. Come on, wise up. Think!
My coming second and third cosmology books will explain the ultimate origin and nature of Dark Matter and Dark Energy.
I agree you’ve gotten it all wrong, but for other reasons. Check out the YouTubes “Dark Matter – A String Theory Way” and “Dark Energy – A String Theory Way”.
Dark matter and dark energy are just lame excuses made by physicists who can’t explain why their models don’t work.
They are theories based on a set of very questionable postulates. That’s not only a simple explanation for what is called Dark Matter and Dark Energy, but it’s a very good thing to remember about any scientific finding. There is no such thing as a scientific fact. Although a lot of people would like to believe things scientists say are facts, they’re not. If you want something to believe, go to church. Dark Matter and Dark Energy are some of the most dubious scientific theories that came out of the late 20th Century.
Dark matter and dark energy are sciences mad scramble to not be wrong. You see without these theorized things there .O’Dell of gravity does not work math.atically. so they invented these things as variables to make the math work.
Too many uneducated people in these comments
Excellent article. Great reading.
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Physics library
Course: physics library > unit 10.
- Specific heat and latent heat of fusion and vaporization
Thermal conduction, convection, and radiation
- Thermal conduction
- Thermal conductivity of metal and wood
- Intuition behind formula for thermal conductivity
- What is thermal conductivity?
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript

IMAGES
VIDEO
COMMENTS
wave. a repeating disturbance that transfers energy through matter or space. medium. matter through which a wave travels. mechanical waves. waves that can travel only through matter. transverse wave. particles in the medium moving back and forth at the right angles to the direction that the wave travels. longitudinal wave.
Some waves can only travel through a material, or medium, such as air or water. These are called mechanical waves. ... In physics, a mechanical wave is a wave that is an oscillation of matter, and therefore transferes energy through a medium.While waves can move over long distances, the movement of the medium of transmission—the material—is ...
The energy from your jump moved across the puddle, but the matter (the molecules of water) only rocked back and forth. Light, or electromagnetic radiation, also can be described as a wave. The energy of light travels through a medium called an electromagnetic field. This field exists everywhere in the universe.
A wave is a disturbance that travels or propagates from the place where it was created. Waves transfer energy from one place to another, but they do not necessarily transfer any mass. Light, sound, and waves in the ocean are common examples of waves. Sound and water waves are mechanical waves; meaning, they require a medium to travel through.
A wave as being made of simple harmonic oscillators. Consider a wave that is propagating through a medium. We can model the motion of one of the particles in the medium as if it were the motion of a simple harmonic oscillator 1.This is illustrated in Figure \(\PageIndex{1}\), which shows the displacement as a function of time for a point in the medium located at the origin when a wave passes ...
It can travel through a vacuum. It always moves at a constant speed, known as the speed of light, which is 300,000,000 meters (186,000 miles) per second in a vacuum. ... Visible light makes up only a tiny slice of this range. DrSciComm/Wikimedia ... Classical physics is an explanation of the nature and properties of matter and energy that ...
An electron volt is the amount of kinetic energy needed to move an electron through one volt potential. Moving along the spectrum from long to short wavelengths, energy increases as the wavelength shortens. Consider a jump rope with its ends being pulled up and down. More energy is needed to make the rope have more waves.
Resource. Add to collection. In electromagnetic waves, energy is transferred through vibrations of electric and magnetic fields. In sound waves, energy is transferred through vibration of air particles or particles of a solid through which the sound travels. In water waves, energy is transferred through the vibration of the water particles.
Examples include gamma rays, X-rays, ultraviolet waves, visible light, infrared waves, microwaves, and radio waves. Electromagnetic waves can travel through a vacuum at the speed of light, v = c = 2.99792458 × 10 8 m/s. v = c = 2.99792458 × 10 8 m/s. For example, light from distant stars travels through the vacuum of space and reaches Earth.
Planck observed that matter actually absorbed or emitted energy only in whole-number multiples of the value h ν , where h is Planck's constant, 6.626 × 10 − 34 J ⋅ s , and ν is the frequency of the light absorbed or emitted. This was a shocking discovery, because it challenged the idea that energy was continuous, and ...
A mechanical wave is a disturbance or oscillation that travels through matter (medium), transferring energy from one point to another. Unlike electromagnetic waves which can travel through a vacuum, mechanical waves rely on particles in a medium to transport their energy. Mechanical Waves and Matter
However, unlike a mechanical transverse wave, which can only travel through matter, an electromagnetic transverse wave can travel through empty space. When waves travel through matter, they lose some energy to the matter as they pass through it. But when waves travel through space, no energy is lost. Therefore, electromagnetic waves don't get ...
The energy of a mechanical wave can travel only through matter. The matter through which the wave travels is called the. What is a Mechanical Wave? A mechanical wave is a disturbance in matter that transfers energy through the matter. A mechanical wave starts when matter is disturbed. A source of energy is needed to disturb matter and start a ...
In nature, energy transfers and transformations happen constantly, such as in a coastal dune environment. When thermal energy radiates from the sun, it heats both the land and ocean, but water has a specific high heat capacity, so it heats up slower than land. This temperature difference creates a convection current, which then manifests as wind.
The movement of energy and matter in ecosystems. Energy flows through an ecosystem, while matter cycles within it. To understand why this is the case let's take a closer look at how different life processes drive the movement of energy and matter in ecosystems. Energy enters an ecosystem when producers carry out photosynthesis, capturing ...
These waves can travel through a vacuum at a constant speed of 2.998 × × 10 8 m/s, the speed of light (denoted by c). All waves, including forms of electromagnetic radiation, are characterized by, a wavelength (denoted by λ , the lowercase Greek letter lambda), a frequency (denoted by ν , the lowercase Greek letter nu), and an amplitude .
September 30, 2016 at 6:15 am. Throughout the universe, it's natural for energy to flow from one place to another. And unless people interfere, thermal energy — or heat — naturally flows in one direction only: from hot toward cold. Heat moves naturally by any of three means. The processes are known as conduction, convection and radiation.
The Medium The energy of a mechanical wave can travel only through matter. The matter through which the wave travels is called the medium (plural, media). The medium in the water wave pictured above is water, a liquid. But the medium of a mechanical wave can be any state of matter, even a solid.
Radiation can even move heat through the emptiness of space. Nearly all thermal energy on Earth comes from the sun. It travels in the form of energy waves, such as light. Materials on Earth take in these waves. They use them for energy or reflect them into space. Energy Can Change Form Energy can also change form. This is called transformation.
Explore the energy and matter cycles found within the Earth System. Energy Cycle. Energy from the Sun is the driver of many Earth System processes. This energy flows into the Atmosphere and heats this system up It also heats up the Hydrosphere and the land surface of the Geosphere, and fuels many processes in the Biosphere.
From what scientists can tell, visible matter makes up only 5% of the universe. Dark matter and dark energy are believed to make up the other 27% and 68%, respectively. In other words, what we know well — visible matter — doesn't even come close to explaining the nature of the vast majority of the universe.
Solar eclipses occur when the sun, moon and Earth align. The moon passes between Earth and sun, temporarily blocking the sun's light and casting a shadow on Earth. A total solar eclipse is when ...
Sound travels as waves of energy, but, unlike light, the waves transmit energy by changing the motion of particles. ... Sound waves can only travel in space if there are enough particles around to transmit the energy in the wave from the source to the listener. If you talk under water, it sounds funny because the water is carrying the sound ...
A total solar eclipse occurs when the moon passes between Earth and the sun, completely blocking the sun's face. Those within the path of totality will see a total solar eclipse. People outside ...
NASA also said in its statement, "Traveling towards the center of the path of totality — even a mile or two — will quickly increase the length of totality that people can see.". And ...
About. Transcript. There are three forms of thermal energy transfer: conduction, convection, and radiation. Conduction involves molecules transferring kinetic energy to one another through collisions. Convection occurs when hot air rises, allowing cooler air to come in and be heated. Thermal radiation happens when accelerated charged particles ...
It will take 1 hour and 8 minutes for the moon's shadow to traverse the country from Texas to Maine, crossing parts of 15 states. The total eclipse darkened the skies in Kerrville, Texas, where ...
Updated April 8, 2024, 2:00 AM PDT. By Denise Chow. Eclipse day has arrived! A total solar eclipse — nicknamed the Great American Eclipse for its long path over North America — will be visible ...