
Finding Hope and Healing: Mindy’s Journey Through Breast Cancer
Meet Mindy, an emotional contributor at Learn Look Locate, who bravely shares her breast cancer journey to inspire and support others. Diagnosed with triple positive stage 2 invasive ductal carcinoma at 29, Mindy turned her battle into a beacon of hope for many facing similar challenges. Join us as we delve into her story, filled with resilience, healing, the pursuit of joy after cancer, and connecting survivors worldwide.
The Diagnosis That Changed Everything
I was diagnosed in early 2016 with breast cancer (triple positive stage 2 invasive ductal carcinoma), already in one lymph node at diagnosis. I was 29 and a competitive runner at the time.
Relating with those around me suddenly became out of reach. Many friends were getting married and having babies, yet there I was in treatment for cancer, the visions my then-fiancé (now husband) and I had for our future blurred beyond recognition.
Navigating Life’s Unexpected Turns
So I began to carve out this little space where I have been sharing my story and my reflections for a number of years now!
As difficult as it can be to revisit these difficult life events, I’ve found writing helps me to process and let my emotions flow through, out, and around instead of pooling in my heart.
Life Beyond Cancer
I no longer have cancer, as far as we know. I completed my 5 years of post-active treatment endocrine therapies in 2021 and my husband and I are hopeful to be able to begin our family soon, but in the meantime, we have loved growing and nurturing the many sweet little babes on our farm here in Missouri!
Professional Journey and Passion
I am a speech-language pathologist and I am in my 12th year of practice.
My passion to continue to share my story to the newly diagnosed and those who will be diagnosed in 2 days, 2 weeks, or 2 years—the men and women who find themselves dramatically removed from the lives they know, thrust into the frightening and often isolating world of cancer and it’s after-effects.
A Space of Comfort and Understanding with Learn Look Locate
In her cherished space, Mindy dreams of creating a soft landing for those affected by breast cancer. Through Learn Look Locate, she extends her reach, embodying the power of shared experiences and the beauty of connection. Check out letters from Mindy as she shares about her journey in details. Mindy’s journey is a testament to the strength found in vulnerability and the healing power of community.
Thank you for being here, in a space where every story matters, and every voice brings us closer to understanding, healing, and hope.
Share This Story, Choose Your Platform!
Educate. inspire. connect..
Sign up for our newsletter.
Information
Learn look locate, llc | © copyright 2023 | all rights reserved | privacy policy | terms & conditions, your journey.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Stage 2 Breast Cancer Diagnosis, Treatment, and Survival Rate
Survival rate for women with stage 2 breast cancer is around 93% with treatment
- Survival Rates
Follow-Up Care
Stage 2 breast cancer survival rates are high and with the right treatment, the outlook is very good. Stage 2 breast cancer means your tumor is at least 1 centimeter (cm) in size and has spread to lymph nodes.
Treatment of stage 2 breast cancer usually includes surgery (either a lumpectomy or mastectomy), and adjuvant chemotherapy (therapy after the initial treatment) is often recommended. Radiation therapy is needed after a lumpectomy, but may or may not be needed after a mastectomy.
This article discusses the diagnosis and treatment of stage 2 breast cancer. It also looks at survival rates for people who are diagnosed at this stage.
Stage 2 breast cancer is considered invasive, meaning that cancer cells have broken out of the ducts or lobules of the breast. This is not the same as metastatic (stage 4) breast cancer. It means that abnormal cells have passed through a thin layer of tissue called the basement membrane and have the potential to spread.
Breast Cancer Treatment Options
Cancers are scored and divided into stages by the TNM system . Stage 2 cancer can be either 2A or 2B.
In this system:
- T stands for tumor size: In stage 2, the T score can range from 0 to 3. T0 means that a tumor cannot be detected in the breast. T1 includes tumors that have a diameter of 2 cm or less (an inch or less). T2 includes tumors that are between 2 and 5 cm in diameter. T3 includes tumors larger than 5 cm in diameter.
- N stands for lymph node involvement: Stage 2 can be either 0 or 1. N0 (N-zero) would mean that cancer has not spread to any lymph nodes. N1mi describes cancers that have spread to lymph nodes but the spread can only be detected microscopically ( micrometastases ). N1 is used to describe tumors that have spread to at least one lymph node near the tumor.
- M stands for metastasis: All stage 2 cancer is M0, meaning no metastases are present.
Your treatment can include a combination of approaches. Treatment options include:
Local Treatments
Surgical choices will include a lumpectomy or a mastectomy. If you opt for a mastectomy, it will also be important to consider the pros and cons of having a single vs. a double mastectomy .
Radiation therapy may be used after a lumpectomy to mitigate the risk of cancer cells recurring in the same breast or nearby lymph nodes. If radiation is recommended, that will affect the timing of any breast reconstruction that you may have.
After a mastectomy, an oncologist may determine that radiation is necessary if the tumor was larger than 5 cm, if there was lymph node involvement, or if cancer was found outside of surgical margins.
Systemic Treatments (Adjuvant)
These therapies will affect your whole body and will help prevent a recurrence. Depending on your age, general health, hormone receptor status, lymph node involvement, and HER2 testing results, you may be given:
- Chemotherapy
- Hormonal therapy including either tamoxifen or an aromatase inhibitor
- A HER2-targeted therapy such as Herceptin
With triple-negative breast cancer, immunotherapy can sometimes be part of systemic therapy. The drug Lynparza (olaparib), a PARP inhibitor, is now being used as part of systemic therapy to treat early-stage and metastatic HER2-negative breast cancer with a BRCA1 or BRCA2 mutation that has previously been treated with chemotherapy—either before or after surgery.
Neoadjuvant Treatment
Your oncologist may suggest systemic treatments before surgery to shrink the tumor. When this approach is successful, the smaller tumor can be removed and local treatment may be given if needed.
Some tumors don't respond well to pre-treatment; when that happens, a mastectomy will be necessary. You may then consider breast reconstruction .
You may spend three to 18 months or longer in the active treatment of stage 2 breast cancer. It may range from surgery and six weeks of radiation to a full array of chemo, radiation, and biologic therapies.
If the tumor is estrogen receptor-positive, hormone therapy is usually prescribed for five to 10 years. For those who are postmenopausal, bisphosphonate therapy may be recommended as well to reduce the risk of recurrence. If the cancer is HER2-positive, targeted therapy is often used prior to surgery.
Survival Rates for Stage 2 Breast Cancer
Stage 2 breast cancer survival rates are high, but it is important to understand that the rates are not a direct indication of how long you will live following the diagnosis and treatment of breast cancer. Rather, rates reflect how many people on average will be expected to survive for a given period of time.
The five-year survival rate for stage 2 breast cancer is 93% for women who have completed treatment. Women with stage 3 cancer have a five-year survival rate of 75%.
Treatments continue to improve over time, so you may have a longer-term estimated survival if you are newly diagnosed.
Is Stage 2 Breast Cancer Curable?
Stage 2 breast cancer is curable with the right treatment. Although recurrence is possible, the chances can be reduced with radiation treatment and appropriate follow-up care.
After your treatment is completed, you will have a five-year minimum follow-up period with your oncologist; check-ups will take place every three then every six months.
During this time, you may need to take hormone therapy if your tumor was hormone-sensitive. Sometimes hormone therapy is recommended beyond five years for those with estrogen receptor-positive tumors.
If your tumor is estrogen-receptor positive, your oncologist may also recommend that you use bisphosphonate therapy if you are postmenopausal. Bisphosphonates are medications that are used for the treatment of osteoporosis, and they also may help reduce the chance that breast cancer will spread to bones (the most common site of metastases).
What are the chances of breast cancer recurrence after treatment for stage 2 breast cancer?
For women diagnosed with stage 2 breast cancer, the 15-year rate of local recurrence is about 16%.
Distant recurrence in those who had a mastectomy is most influenced by axillary lymph node involvement. When axillary lymph nodes are not cancerous, the recurrence rate is 6% in five years. When axillary lymph nodes are cancerous, the recurrence rate is 23% in five years with mastectomy but no radiation.
Unlike some cancers, routine scans are not usually done after primary treatment for stage 2 breast cancer has been completed. The reason for this, even though recurrence is a possibility, is that finding a recurrence early (before symptoms appear) does not improve survival. For those who have finished treatment, it's important to be familiar with the potential signs and symptoms of a recurrence and to contact your healthcare provider with any concerns.
After five years, you may only need to see your oncologist annually, but these visits most often continue throughout your life. Check-ups are important to make sure that recovery is going smoothly and that treatment for recurrence won't be needed.
Breast Cancer Healthcare Provider Discussion Guide
Get our printable guide for your next healthcare provider's appointment to help you ask the right questions.
Sign up for our Health Tip of the Day newsletter, and receive daily tips that will help you live your healthiest life.
Thank you, {{form.email}}, for signing up.
There was an error. Please try again.
If you've been recently diagnosed with stage 2 breast cancer, you may feel overwhelmed.
There is a multitude of resources for receiving support and learning more about your diagnosis. Ask for help and reach out to your loved ones. Consider becoming involved in one of the active breast cancer support groups or support communities available.
Most importantly, learn how to be your own advocate in your cancer care . Cancer treatments are changing rapidly, and it's important to understand the options available so you can be an active member of your cancer treatment team.
If you've been diagnosed with stage 2 breast cancer, the outlook is very good. You are more likely to have chemotherapy and/or radiation therapy than if your tumor was stage 1, but these tumors are still very treatable.
If your cancer is successfully treated, you will need follow-up care, which includes treatment that decreases the chances of a cancer recurrence.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer . Biol Res . 2017;50(1):33.doi:10.1186/s40659-017-0140-9
Marta GN, Poortmans PM, Buchholz TA, Hijal T. Postoperative radiation therapy after nipple-sparing or skin-sparing mastectomy: A survey of European, North American, and South American practices . Breast J . 2017;23(1):26-33. doi:10.1111/tbj.12683
Cancer: What do the codes in the doctor's letter mean? InformedHealth.org [Internet].
Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer-major changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual . CA Cancer J Clin . 2017;67(4):290-303. doi:10.3322/caac.21393
Koh J, Kim MJ. I ntroduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage . Korean J Radiol . 2019;20(1):69–82. doi:10.3348/kjr.2018.0231
Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer . Breast Cancer Res Treat . 2018;170(3):647–656. doi:10.1007/s10549-018-4796-9
Yao K, Sisco M, Bedrosian I. Contralateral prophylactic mastectomy: current perspectives . Int J Womens Health . 2016;8:213–223. doi:10.2147/IJWH.S82816
Lee SH, Kim YS, Han W, et al. Tumor growth rate of invasive breast cancers during wait times for surgery assessed by ultrasonography . Medicine (Baltimore). 2016;95(37):e4874. doi:10.1097/MD.0000000000004874
Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer . Cancer . 2020;126(19):4278-4288. doi:10.1002/cncr.33102
Breastcancer.org. Lynparza .
Teven CM, Schmid DB, Sisco M, Ward J, Howard MA. Systemic Therapy for Early-Stage Breast Cancer: What the Plastic Surgeon Should Know . Eplasty . 2017;17:e7. PMID: 28293332
Wimmer K, Strobl S, Bolliger M, et al. Optimal duration of adjuvant endocrine therapy: how to apply the newest data . Ther Adv Med Oncol . 2017;9(11):679–692. doi:10.1177/1758834017732966
Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022 . CA Cancer J Clin . 2022;72(6):524-541. doi:10.3322/caac.21754
Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations . Perm J . 2015;19(2):48–79. doi:10.7812/TPP/14-241
Gupta S, Singh M, Vora A, et al. Practical consensus recommendations on duration of adjuvant hormonal therapy in breast cancer . South Asian J Cancer . 2018;7(2):142–145. doi:10.4103/sajc.sajc_122_18
Wang L, Zhang S, Wang X. The metabolic mechanisms of breast cancer metastasis . Front Oncol . 2021;10:602416. doi:10.3389/fonc.2020.602416
Sopik V, Nofech-Mozes S, Sun P, Narod SA. The relationship between local recurrence and death in early-stage breast cancer . Breast Cancer Res Treat . 2016;155(1):175-85. doi:10.1007/s10549-015-3666-y
Carlson RW. Chapter 67: Surveillance of patients following primary therapy. In Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast, 5th edition . Lippincott Williams and Wilkins, 2014.
American Cancer Society. Follow-up care after breast cancer treatment .
Urquhart R, Lethbridge L, Porter GA. Patterns of cancer centre follow-up care for survivors of breast, colorectal, gynecologic, and prostate cancer . Curr Oncol . 2017;24(6):360–366. doi:10.3747/co.24.3627
Su JA, Yeh DC, Chang CC, et al. Depression and family support in breast cancer patients . Neuropsychiatr Dis Treat . 2017;13:2389–2396. doi:10.2147/NDT.S135624
Hagan TL, Medberry E. Patient Education vs. Patient Experiences of Self-advocacy: Changing the Discourse to Support Cancer Survivors . J Cancer Educ . 2016;31(2):375–381. doi:10.1007/s13187-015-0828-x
Scharl A, Kühn T, Papathemelis T, Salterberg A. The right treatment for the right patient - Personalised treatment of breast cancer . Geburtshilfe Frauenheilkd . 2015;75(7):683–691. doi:10.1055/s-0035-1546270
National Cancer Institute (US). Breast Cancer Treatment (Adult) (PDQ®) . PDQ Cancer Information Summaries [Internet].
By Lynne Eldridge, MD Lynne Eldrige, MD, is a lung cancer physician, patient advocate, and award-winning author of "Avoiding Cancer One Day at a Time."
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
Breast Cancer Stages and Staging
Breast cancer stage.
Breast cancer stage describes the extent of the cancer within your body.
The stage of your breast cancer helps plan your treatment.
Breast cancer stage is the most important factor for prognosis (chance of survival). In general, the earlier the stage, the better the prognosis will be.
Breast cancer staging
Pathologic staging is the standard way to stage breast cancer. It’s based on a pathologist’s study of the tumor tissue and any lymph nodes removed during surgery.
Clinical staging includes results from a health care provider’s physical exam, tests and/or imaging, such as mammography. Sometimes, these findings may add to the pathologist’s findings and may help with staging.
TNM system of staging
The main method of pathologic staging for breast cancer is the TNM system.
TNM stands for:
- T = T umor size
- N = Lymph N ode status (the number and location of lymph nodes with cancer)
- M = M etastases (whether or not the cancer has spread to other parts of the body)
A “p” before the T or N shows these are pathology findings from the tumor or lymph nodes removed during surgery.
In the past, tumor stage was classified using only the 3 TNM measures. Starting in 2018, the TNM system added these measures:
- Tumor grade
- Estrogen receptor status
- Progesterone receptor status
- HER2 status
Learn more about tumor size (T) and staging .
Learn more about lymph node status (N) and staging .
Learn more about metastases (M) and staging .
Why were new measures added to the staging system?
The new measures give information on the biology of the tumor that affects prognosis. Adding these measures improved staging.
For example, with breast cancer, a large tumor may have a better prognosis than a small tumor based on biological measures. In the same way, a small tumor may have a worse prognosis than a large tumor based on these measures.
What if I was diagnosed with breast cancer before 2018?
If you were diagnosed before 2018, your breast cancer was staged a bit differently than it would be today.
Find information on breast cancer staging before 2018 .
Neoadjuvant therapy and breast cancer staging
Neoadjuvant therapy is treatment, such as chemotherapy , HER2-targeted therapy , hormone therapy or immunotherapy , given before surgery . If you will get neoadjuvant therapy, your breast cancer will be staged differently from someone who has surgery as their first treatment.
Neoadjuvant therapy can shrink tumors in the breast and lymph nodes, changing the original tumor size and lymph node status. So, your breast cancer is staged using information from physical exams, imaging and biopsies done before neoadjuvant therapy, rather than information from the tumor removed during surgery.
The stages shown in the table below are only used to classify breast cancers in people who have surgery as their first treatment.
If you will get neoadjuvant therapy, talk with your health care provider about how your breast cancer will be staged.
Learn about information on a pathology report for people who get neoadjuvant therapy .
Stages of breast cancer
The stages of breast cancer range from 0 to IV (0 to 4).
The highest stage (stage IV) is any breast cancer with metastases (M1), no matter the size of the tumor, the lymph node status or other factors. This is known as metastatic breast cancer and is the most advanced stage of disease.
Most often, the higher the stage of the cancer, the poorer the prognosis (chance of survival) will be.
The table below lists the TNM classifications for each stage of breast cancer for people who have surgery as their first treatment.
The following is a 3D interactive model showing the stages of breast cancer from 0 to IV. Click the arrows to move through the model to learn more about breast cancer .
Oncotype DX ® and breast cancer stage
Oncotype DX is part of staging for some estrogen receptor-positive , lymph node-negative early breast cancers.
Learn more about Oncotype DX .
Updated 12/21/23
TOOLS & RESOURCES
Breast Cancer Prognosis
Interactive Learning
Breast Cancer 101 – Types and Staging
- About Breast Cancer
- Find Support
- Get Involved
- Free Resources
- Mammogram Pledge
- Wall of Support
- In The News
- Recursos en Espa ñ ol
About Breast Cancer > Stages > Stage 2 (II) And Stage 2A (IIA) Breast Cancer Overview
- What Is Cancer?
- Causes of Breast Cancer
- Breast Cancer Facts & Stats
- Breast Tumors
- Breast Anatomy
- Male Breast Cancer
- Growth of Cancer
- Risk Factors
- Genetic Testing for Breast Cancer
- Other Breast Cancer Genes
- BRCA: The Breast Cancer Gene
- What To Do If You Tested Positive
- Breast Cancer Signs and Symptoms
- Breast Lump
- Breast Pain
- Breast Cyst
- Breast Self-Exam
- Clinical Breast Exam
- How to Schedule a Mammogram
- Healthy Habits
- Breast Cancer Screening
- Diagnostic Mammogram
- Breast Biopsy
- Waiting For Results
- Breast Cancer Stages
- Stage 2 (II) And Stage 2A (IIA)
- Stage 3 (III) A, B, And C
- Stage 4 (IV) Breast Cancer
- Ductal Carcinoma In Situ (DCIS)
- Invasive Ductal Carcinoma (IDC)
- Lobular Carcinoma In Situ (LCIS)
- Invasive Lobular Cancer (ILC)
- Triple Negative Breast Cancer (TNBC)
- Inflammatory Breast Cancer (IBC)
- Metastatic Breast Cancer (MBC)
- Breast Cancer During Pregnancy
- Other Types
- Choosing Your Doctor
- Lymph Node Removal & Lymphedema
- Breast Reconstruction
- Chemotherapy
- Radiation Therapy
- Hormone Therapy
- Targeted Therapy
- Side Effects of Breast Cancer Treatment and How to Manage Them
- Metastatic Breast Cancer Trial Search
- Standard Treatment vs. Clinical Trials
- Physical Activity, Wellness & Nutrition
- Bone Health Guide for Breast Cancer Survivors
- Follow-Up Care
- Myth: Finding a lump in your breast means you have breast cancer
- Myth: Men do not get breast cancer; it affects women only
- Myth: A mammogram can cause breast cancer or spread it
- Myth: If you have a family history of breast cancer, you are likely to develop breast cancer, too
- Myth: Breast cancer is contagious
- Myth: If the gene mutation BRCA1 or BRCA2 is detected in your DNA, you will definitely develop breast cancer
- Myth: Antiperspirants and deodorants cause breast cancer
- Myth: A breast injury can cause breast cancer
- Myth: Breast cancer is more common in women with bigger breasts
- Myth: Breast cancer only affects middle-aged or older women
- Myth: Breast pain is a definite sign of breast cancer
- Myth: Consuming sugar causes breast cancer
- Myth: Carrying a phone in your bra can cause breast cancer
- Myth: All breast cancers are the same
- Myth: Bras with underwire can cause breast cancer
- Can physical activity reduce the risk of breast cancer?
- Can a healthy diet help to prevent breast cancer?
- Does smoking cause breast cancer?
- Can drinking alcohol increase the risk of breast cancer?
- Is there a link between oral contraceptives and breast cancer?
- Is there a link between hormone replacement therapy (HRT) and breast cancer?
- How often should I do a breast self exam (BSE)?
- Does a family history of breast cancer put someone at a higher risk?
- Are mammograms painful?
- How does menstrual and reproductive history affect breast cancer risks?
- How often should I go to my doctor for a check-up?
- What kind of impact does stress have on breast cancer?
- What celebrities have or have had breast cancer?
- Where can I find a breast cancer support group?
- Can breastfeeding reduce the risk of breast cancer?
- Is dairy (milk) linked to a higher risk of breast cancer?
- Is hair dye linked to a higher risk of breast cancer?
- NEW! Just Diagnosed with Breast Cancer… Now What?
- Smart Bites Cookbook: 7 Wholesome Recipes in 35 Minutes (or Less!)
- Weekly Healthy Living Tips: Volume 2
- Most Asked Questions: Breast Cancer Signs & Symptoms
- Cancer Caregiver Guide
- Breast Cancer Surgery eBook
- 10 Prompts to Mindfulness
- How to Talk About Breast Health
- Family Medical History Checklist
- Healthy Recipes for Cancer Patients eBook
- Chemo Messages
- Most Asked Questions About Breast Cancer Recurrence
- Breast Problems That Arent Breast Cancer eBook
- Nutrition Care for Breast Cancer Patients eBook
- Finding Hope that Heals eBook
- Dense Breasts Q&A Guide
- Breast Cancer Recurrence eBook
- What to Say to a Cancer Patient eBook
- Weekly Healthy Living Tips
- Bra Fit Guide
- Know the Symptoms Guide
- Breast Health Guide
- Mammogram 101 eBook
- 3 Steps to Early Detection Guide
- Abnormal Mammogram eBook
- Healthy Living & Personal Risk Guide
- What Every Woman Needs to Know eBook
- Breast Cancer Resources
Stage 2 (II) And Stage 2A (IIA) Breast Cancer Overview

Last updated on Jan 17, 2024
What Does It Mean To Have Stage 2 Breast Cancer?
Stage 2 means the breast cancer is growing, but it is still contained in the breast or growth has only extended to the nearby lymph nodes.
This stage is divided into groups: Stage 2A and Stage 2B. The difference is determined by the size of the tumor and whether the breast cancer has spread to the lymph nodes.
For Stage 2 breast cancer, chemotherapy is usually done first, followed by surgery and radiation therapy.
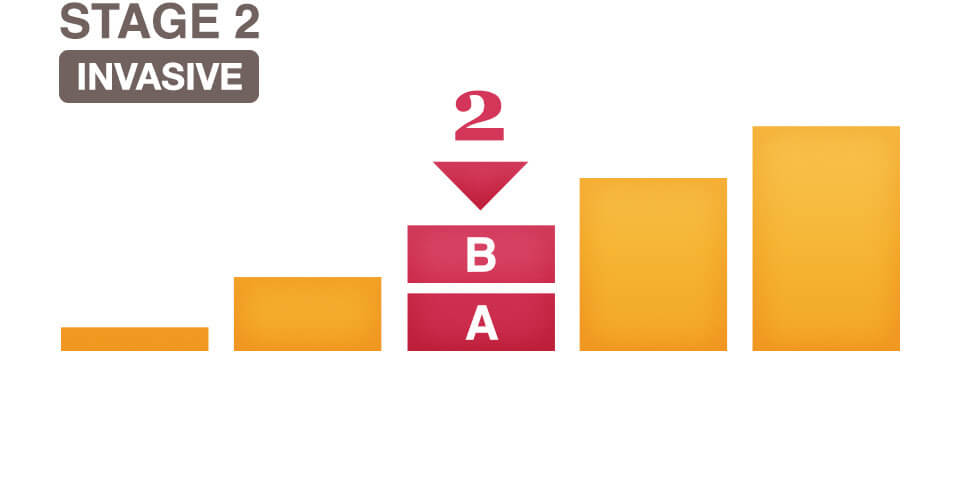
Stage IIA Breast Cancer Means One Of The Following Descriptions Applies.
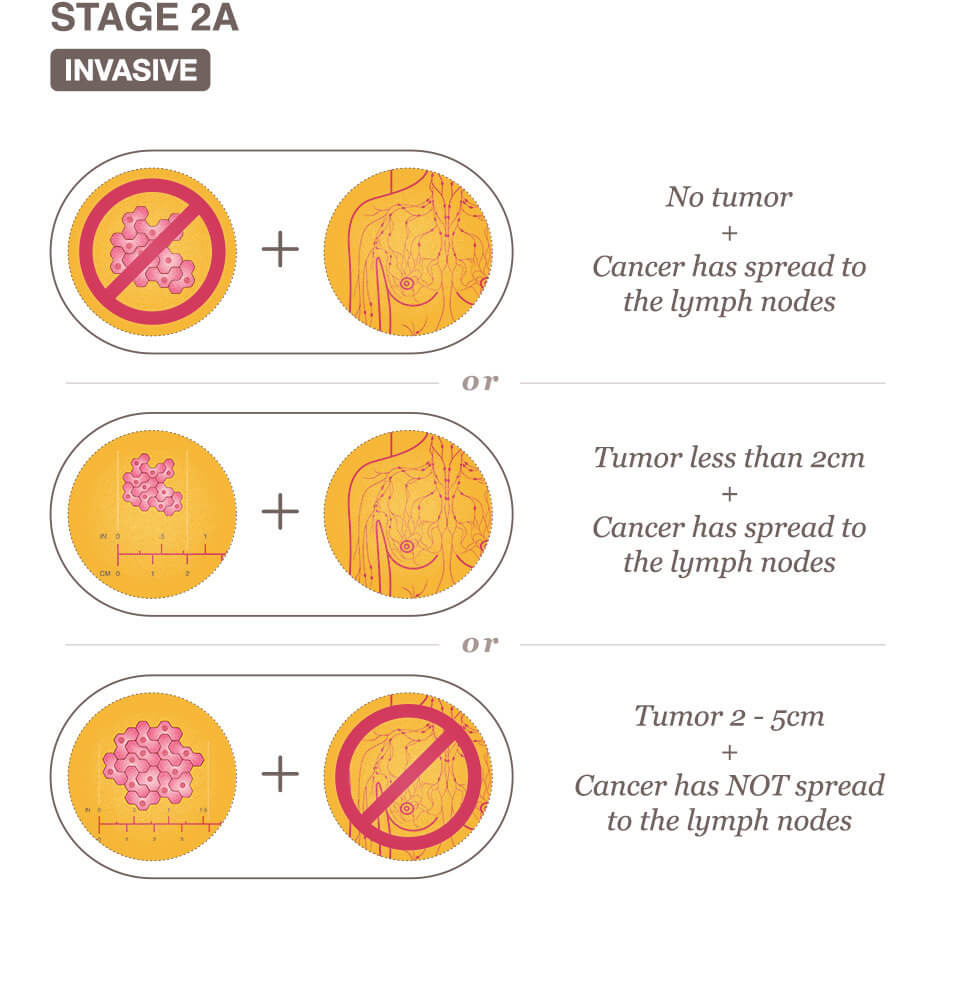
No actual tumor is associated with the cancerous cells and less than four axillary lymph nodes have cancer cells present.
The tumor is less than 2 centimeters and less than four axillary lymph nodes have cancer cells present.
The tumor is between 2 and 5 centimeters and has not yet spread to the lymph nodes.
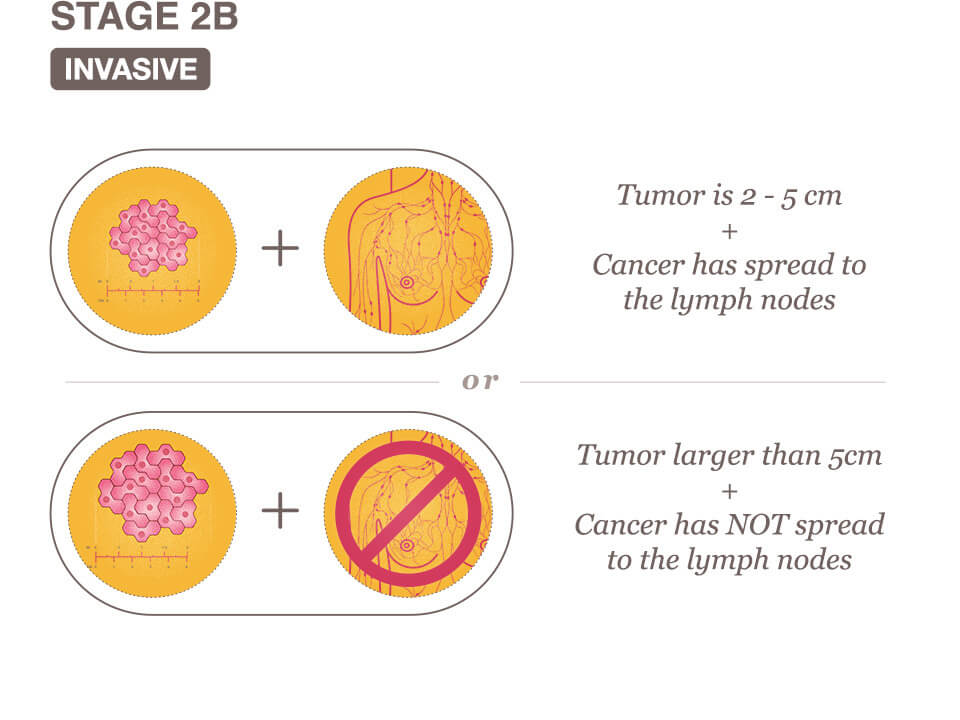
Stage IIB Breast Cancer Means One Of The Following Descriptions Applies.
The tumor is between the 2 and 5 centimeters and has spread to less than four axillary lymph nodes.
The tumor is larger than five centimeters, but has not spread to any axillary lymph nodes.
Related reading:
- Stage 0 Breast Cancer Overview
- Stage 1 Breast Cancer Overview
- Stage 3 (III) A, B, And C Breast Cancer Overview
We use cookies on our website to personalize your experience and improve our efforts. By continuing, you agree to the terms of our Privacy & Cookies Policies.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Breast cancer patient experiences through a journey map: A qualitative study
Laura ciria-suarez.
1 Clinical Psychology and Psychobiology Department, Faculty of Psychology, University of Barcelona, Barcelona, Spain
Paula Jiménez-Fonseca
2 Medical Oncology Department Hospital Universitario Central of Asturias, Oviedo, Spain
María Palacín-Lois
3 Social Psychology and Quantitative Psychology Department, Faculty of Psychology, University of Barcelona, Barcelona, Spain
Mónica Antoñanzas-Basa
4 Medical Oncology Department, Hospital Universitario Clínico San Carlos, Madrid, Spain
Ana Fernández-Montes
5 Medical Oncology Department, Complexo Hospitalario Universitario de Ourense, Ourense, Spain
Aranzazu Manzano-Fernández
Beatriz castelo.
6 Medical Oncology Department, Hospital Universitario La Paz, Madrid, Spain
Elena Asensio-Martínez
7 Medical Oncology Department, Hospital General Universitario de Elche, Elche, Spain
Susana Hernando-Polo
8 Medical Oncology Department, Hospital Universitario Fundación Alcorcón, Madrid, Spain
Caterina Calderon
Associated data.
Relevant anonymized data excerpts from the transcripts are in the main body of the manuscript. They are supported by the supplementary documentation at 10.1371/journal.pone.0244355 .
Breast cancer is one of the most prevalent diseases in women. Prevention and treatments have lowered mortality; nevertheless, the impact of the diagnosis and treatment continue to impact all aspects of patients’ lives (physical, emotional, cognitive, social, and spiritual).
This study seeks to explore the experiences of the different stages women with breast cancer go through by means of a patient journey.
This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho-oncologists. Data were processed by adopting a thematic analysis approach.
The diagnosis and treatment of breast cancer entails a radical change in patients’ day-to-day that linger in the mid-term. Seven stages have been defined that correspond to the different medical processes: diagnosis/unmasking stage, surgery/cleaning out, chemotherapy/loss of identity, radiotherapy/transition to normality, follow-up care/the “new” day-to-day, relapse/starting over, and metastatic/time-limited chronic breast cancer. The most relevant aspects of each are highlighted, as are the various cross-sectional aspects that manifest throughout the entire patient journey.
Conclusions
Comprehending patients’ experiences in depth facilitates the detection of situations of risk and helps to identify key moments when more precise information should be offered. Similarly, preparing the women for the process they must confront and for the sequelae of medical treatments would contribute to decreasing their uncertainty and concern, and to improving their quality-of-life.
Introduction
Breast cancer is the most common cancer and the one that associates the highest mortality rates among Spanish women, with 32,953 new cases estimated to be diagnosed in Spain in 2020 [ 1 ]. Thanks to early diagnosis and therapeutic advances, survival has increased in recent years [ 2 ]. The 5-year survival rate is currently around 85% [ 3 , 4 ].
Though high, this survival rate is achieved at the expense of multiple treatment modalities, such as surgery, chemotherapy, radiotherapy, and hormone therapy, the side effects and sequelae of which can interfere with quality-of-life [ 5 ]. Added to this is the uncertainty surrounding prognosis; likewise, life or existential crises are not uncommon, requiring great effort to adjust and adapt [ 6 ]. This will not only affect the patient psychologically, but will also impact their ability to tolerate treatment and their socio-affective relations [ 7 ].
Several medical tests are performed (ultrasound, mammography, biopsy, CT, etc.) to determine tumor characteristics and extension, and establish prognosis [ 8 ]. Once diagnosed, numerous treatment options exist. Surgery is the treatment of choice for non-advanced breast cancer; chemotherapy, radiotherapy, and hormone therapy are adjuvant treatments with consolidated benefit in diminishing the risk of relapse and improving long-term survival [ 9 ]. Breast cancer treatments prompt changes in a person’s physical appearance, sexuality, and fertility that interfere with their identity, attractiveness, self-esteem, social relationships, and sexual functioning [ 10 ]. Patients also report more fatigue and sleep disturbances [ 11 ]. Treatment side effects, together with prognostic uncertainty cause the woman to suffer negative experiences, such as stress in significant relationships, and emotions, like anxiety, sadness, guilt, and/or fear of death with negative consequences on breast cancer patients’ quality-of-life [ 10 , 12 ]. Once treatment is completed, patients need time to recover their activity, as they report decreased bodily and mental function [ 13 ], fear of relapse [ 14 ], and changes in employment status [ 15 ]. After a time, there is a risk of recurrence influenced by prognostic factors, such as nodal involvement, size, histological grade, hormone receptor status, and treatment of the primary tumor [ 16 ]. Thirty percent (30%) of patients with early breast cancer eventually go on to develop metastases [ 17 ]. There is currently no curative treatment for patients with metastatic breast cancer; consequently, the main objectives are to prolong survival, enhance or maintain quality-of-life, and control symptoms [ 17 , 18 ]. In metastatic stages, women and their families are not only living with uncertainty about the future, the threat of death, and burden of treatment, but also dealing with the existential, social, emotional, and psychological difficulties their situation entails [ 18 , 19 ].
Supporting and accompanying breast cancer patients throughout this process requires a deep understanding of their experiences. To describe the patient’s experiences, including thoughts, emotions, feelings, worries, and concerns, the phrase “patient voice” has been used, which is becoming increasingly common in healthcare [ 20 ]. Insight into this “voice” allows us to delve deeper into the physical, emotional, cognitive, social, and spiritual effects of the patient’s life. This narrative can be portrayed as a “cancer journey", an experiential map of patients’ passage through the different stages of the disease [ 21 ] that captures the path from prevention to early diagnosis, acute care, remission, rehabilitation, possible recurrence, and terminal stages when the disease is incurable and progresses [ 22 ]. The term ‘patient journey’ has been used extensively in the literature [ 23 – 25 ] and is often synonymous with ‘patient pathway’ [ 26 ]. Richter et al. [ 26 ] state that there is no common definition, albeit in some instances the ‘patient journey’ comprises the core concept of the care pathway with greater focus on the individual and their perspective (needs and preferences) and including mechanisms of engagement and empowerment.
While the patient’s role in the course of the disease and in medical decision making is gaining interest, little research has focused on patient experiences [ 27 , 28 ]. Patient-centered care is an essential component of quality care that seeks to improve responsiveness to patients’ needs, values, and predilections and to enhance psychosocial outcomes, such as anxiety, depression, unmet support needs, and quality of life [ 29 ]. Qualitative studies are becoming more and more germane to grasp specific aspects of breast cancer, such as communication [ 27 , 30 ], body image and sexuality [ 31 , 32 ], motherhood [ 33 ], social support [ 34 ], survivors’ reintegration into daily life [ 13 , 15 ], or care for women with incurable, progressive cancer [ 17 ]. Nevertheless, few published studies address the experience of women with breast cancer from diagnosis to follow-up. These include a clinical pathway approach in the United Kingdom in the early 21st century [ 35 ], a breast cancer patient journey in Singapore [ 25 ], a netnography of breast cancer patients in a French specialized forum [ 28 ], a meta-synthesis of Australian women living with breast cancer [ 36 ], and a systematic review blending qualitative studies of the narratives of breast cancer patients from 30 countries [ 37 ]. Sanson-Fisher et al. [ 29 ] concluded that previously published studies had examined limited segments of patients’ experiences of cancer care and emphasized the importance of focusing more on their experiences across multiple components and throughout the continuum of care. Therefore, the aim of this study is to depict the experiences of Spanish breast cancer patients in their journey through all stages of the disease. To the best of our knowledge, there are no studies that examine the experience of women with breast cancer in Spain from diagnosis through treatment to follow-up of survivors and those who suffer a relapse or incurable disease presented as a journey map.
A map of the breast cancer patient’s journey will enable healthcare professionals to learn first-hand about their patients’ personal experiences and needs at each stage of the disease, improve communication and doctor-patient rapport, thereby creating a better, more person-centered environment. Importantly, understanding the transitional phases and having a holistic perspective will allow for a more holistic view of the person. Furthermore, information about the journey can aid in shifting the focus of health care toward those activities most valued by the patient [ 38 ]. This is a valuable and efficient contribution to the relationship between the system, medical team, and patients, as well as to providing resources dedicated to the patient’s needs at any given time, thus improving their quality of life and involving them in all decisions.
Study design and data collection
We conducted a qualitative study to explore the pathway of standard care for women with breast cancer and to develop a schematic map of their journey based on their experiences. A detailed description of the methodology is reported in the published protocol “Ascertaining breast cancer patient experiences through a journey map: A qualitative study protocol” [ 39 ].
An interview guide was created based on breast cancer literature and adapted with the collaboration of two medical oncologists, three nurses (an oncology nurse from the day hospital, a case manager nurse who liaises with the different services and is the ‘named’ point of contact for breast cancer patients for their journey throughout their treatment, and a nurse in charge of explaining postoperative care and treatment), and two psycho-oncologists. The interview covered four main areas. First, sociodemographic and medical information. Second, daily activities, family, and support network. Third, participants were asked about their overall perception of breast cancer and their coping mechanisms. Finally, physical, emotional, cognitive, spiritual, and medical aspects related to diagnosis, treatment, and side effects were probed. Additionally, patients were encouraged to express their thoughts should they want to expand on the subject.
The study was carried out at nine large hospitals located in six geographical areas of Spain. To evaluate the interview process, a pilot test was performed. Interviews were conducted using the interview guide by the principal investigator who had previous experience in qualitative research. Due to the Covid-19 pandemic, all interviews were completed online and video recorded with the consent of the study participants for subsequent transcription. Relevant notes were taken during the interview to document key issues and observations.
Participant selection and recruitment
Inclusion criteria were being female, over 18 years of age, having a diagnosis of histologically-confirmed adenocarcinoma of the breast, and good mental status. To ascertain the reality of women with breast cancer, most of the patients recruited (80%) had been diagnosed in the past 5 years. Patients (20%) were added who had been diagnosed more than 5 years earlier, with the aim of improving the perspective and ascertaining their experience after 5 years.
Medical oncologists and nurses working at the centers helped identify patients who met the inclusion criteria. Participants went to the sites for follow-up between December 2019 and January 2021. Eligible women were informed of the study and invited to participate during an in-person visit by these healthcare professionals. Those who showed interest gave permission to share their contact information (e-mail or telephone number) with the principal investigator, who was the person who conducted all interviews. The principal investigator contacted these women, giving them a more detailed explanation of the study and clarifying any doubts they may have. If the woman agreed to participate, an appointment was made for a videoconference.
A total of 21 women agreed to participate voluntarily in this research. With the objective of accessing several experiences and bolstering the transferability of the findings, selection was controlled with respect to subjects’ stage of cancer, guaranteeing that there would be a proportional number of women with cancer in all stages, as well as with relapses.
Data analysis
The data underwent qualitative content analysis. To assure trustworthiness, analyses were based on the system put forth by Graneheim, and Lundman [ 40 ]. Interviews were transcribed and divided into different content areas; units of meaning were obtained and introduced into each content area; meaning codes were extracted and added; codes were categorized in terms of differences and similarities, and themes were created to link underlying meanings in the categories. All members of the research team (core team, two medical oncologists, three nurses and two psycho-oncologists) reviewed the data and triangulated the outcomes between two sources of data: qualitative data from the interview and non-modifiable information, such as sociodemographic (i.e., age, marital status, having children) and clinical (i.e., cancer stage and surgery type) data. Following this process, we reached saturation of the interview data by the time we had completed 21 interviews.
Ethical considerations
This study was performed in accordance with the ethical standards of the Declaration of Helsinki, and its subsequent amendments. The study was approved by the Research Ethics Committee of University of Barcelona (Institutional Review Board: IRB00003099) and supported by the Bioethics Group of the Spanish Society of Medical Oncology (SEOM) 2018 grant. All participants received a written informed consent form that they signed prior to commencing with the interviews and after receiving information about the study.
Patient baseline characteristics
In total, 21 women with a mean age of 47 years (range, 34 to 61) were interviewed. Most of the study population was married (66.7%), had a college education (66.7%), and had 2 or more children (42.9%). All cancer stages were represented, up to 23.8% tumor recurrence, and most of the primary cancers had been resected (95.2%) (see Table 1 ).
Description of the breast cancer patient journey
The women diagnosed with breast cancer describe the journey as a process tremendously affected by the different medical stages. Each stage has its own characteristics that condition the experiences, unleashing specific physical, emotional, cognitive, and social processes. Additionally, the patients perceive this entire process as pre-established journey they must undertake to save their life, with its protocols based on the type and stage of cancer.
“ People said to me , ‘What do you think ? ’ and I answered that there was nothing for me to think about because everything is done , I have to go on the journey and follow it and wait to see how it goes” (Patient 6)
Fig 1 displays the various phases of the journey that patients with breast cancer go through; nevertheless, each woman will go through some or others, depending on their type of cancer.

Throughout the entire patient journey
Processes of loss and reinterpretation of the new circumstance . What stands out the most in the process these women go through during the diagnosis and treatment of breast cancer is loss; specifically, the loss of health and a reinterpretation of the new circumstance and the new bodily reality. In the most extreme cases, the loss of health emerges with the fear of death that many women report at the time of diagnosis or during treatment, due to the distress generated. The loss of identity seems to be related to the evolutionary (existential) moment in which the woman is; there are patients who report feelings of disability or loss of attractiveness, or fear of not being able to get pregnant in the future, especially the youngest.
I felt a terrifying fear and thought , “You have cancer you tell yourself , you’re going to die tomorrow .” (Patient 6) I feel like after the hysterectomy , as a woman , I no longer have anything , only the physical . Sure , I look great , but I tell myself that it’s just a shell , the shell I inhabit , because as a woman , I only have one breast left . (Patient 6) At that moment , I had to make the decision that I was no longer going to be a mother . (Patient 14)
Personal change . Most of the women report that with the diagnosis of breast cancer, their life stands still and from that point forward, a different journey begins. The sole focus on this journey is the disease and its implications. During all those months, the patients stop working; they focus on their medical treatments, and reflect a lot on their current situation and on life. Most of the participants state, especially those who have already been discharged, that they know themselves better now; they take better care of themselves, and they enjoy their day-to-day and the small moments more, making the most of their time, with more initiatives and fewer trivial complaints.
Clearly , you’re not the same person you were before; I don’t think she’ll ever come back; your mindset changes completely and I have sequelae from all the treatments . (Patient 1) I re-think wasting energy on lost causes; what’s more , I’ve also learnt to say no . If I’m not in the mood to go somewhere , I just say no . (Patient 7) I take much more advantage of the present now , because you realize that things can change on any given day . (Patient 3)
Trust and appreciation for their physician . Most of the interviewees stated that they fully trusted the doctors who care for them, without question or objection to the treatments proposed. They reported that, as they go forward, they discuss the tests and treatments that are going to be performed, as well as possible side effects. Several stated that they are unaware of the stage of their cancer; similarly, most also do not know the benefits expressed in X% of the treatments. A few of the participants claimed that they did talk in detail about the different types of treatments with their oncologists, that they had sought another opinion, and one of them even reported having decided to stop chemotherapy, which was very hard for her, given her physician’s insistence that she continue.
The truth is that the oncologist didn’t say much about percentages; what she told me were the steps that I had to take; I thoroughly trusted her and she gave me a lot of peace of mind . (Patient 5) I told him , “I’m going to do whatever you tell me to . ” It never occurred to me to dispute whatever the oncologist might tell me . I was willing to do whatever was needed . (Patient 8)
Most of the women, at some point during the interview, state that they are grateful for the care they received and that, within the seriousness of their situation, there is a treatment for their condition.
I am super grateful for the treatment I’ve received and with the doctors assigned to me . (Patient 2) I’m very lucky; I’m only on my second line of treatment for metastasis and I’ve got a lot more ahead of me , but I consider myself lucky and I believe things are going very well . (Patient 20)
Role of the woman . We can see that the women adopt a role of care-givers and managers of their surroundings. They worry about the disease negatively affecting the people around them, which is why they make an effort to manage the family’s activity for when they can’t do it and they try to avoid being a physical burden or cause emotional distress to the people around them.
I was very strong ; I made everything easy for people , but making it very easy , doesn’t mean that it was easy for me , but that I made it easy for everyone . (Patient 8) I didn’t want to worry anyone because that’s just the way I am , I push forward and that’s that . (Patient 5)
Support network . In all cases, the family appears to be one of the elements that is most involved in the disease process. Within the family, the partner deserves special mention. The testimonies in this regard reveal a wide spectrum of possibilities that range from the feeling of having had great support to a lack of attention and understanding that, in many situations, causes the relationship to be strained or to end. Friends tend to appear more occasionally.
I can’t complain about my husband; he was up to the challenge , very attentive toward me and he fully understood how I was feeling ; I felt very supported . (Patient 14) We’ve had a period of a lot of arguing; I’ve had to sit down with him and tell him that life had changed for me . (Patient 18) I had a partner I had lived with for five and a half years and he told me , literally , that he looked at me like a little sister , no longer as a woman , and he left me , and that hurt me tremendously . (Patient 6)
On the other hand, many patients commented on the importance of social media, where they have met people in the same situation as them. They report feeling understood and in good company; likewise, they commented on the importance of being able to share their doubts and get to know about other experiences.
It’s a situation that only someone who has gone through can understand; you can have all the good intentions in the world , but if you haven’t gone through it , you can’t even begin to understand . (Patient 8)
Use of complementary treatments . Most patients follow conventional medical treatment. However, many resort to other disciplines that help them improve their quality-of-life, like dietary changes, getting more exercise than usual, visits to a psychologist or physical therapist, or using other integrative therapies, such as acupuncture, yoga, reiki, flowers of Bach, homeopathy, cannabis, or meditation.
I started to read a whole bunch of books to see what I could do to take care of myself in terms of nutrition and exercise ; you consider everything you can do . (Patient 5)
Diagnosis/unmasking
This phase encompasses the time from when the woman detects some symptom or goes to a check-up until the medical diagnosis is made. For the woman, this is a time of a series of tests and results. We have observed that the procedures, especially the healthcare professionals that deal with the patients, and the timing vary, depending on the medical center where they are being cared for. Emotionally, this is one of the most complicated stages.
Emotional whirlwind . The wait to obtain test results has a huge emotional impact for the women, given that it is a time of great uncertainty and fear.
An entire month with all the anguish of finding out if you have something . (Patient 3) The worst part is waiting 15 days to find out the magnitude of the tragedy , if it’s throughout your entire body or only in your breast; you go through a brutal emotional whirlwind; the wait is horrible because there’s nothing else you can do , so that anguish that you carry inside is dreadful; it was hell for me . (Patient 10)
Additionally, the interviewees described many other emotions that included fear of death, fear of having no time, feeling of unreality, rage, anger, sadness, avoidance, denial…
The first thing I thought was that I was going to die and that I wouldn’t finish watching my children [grow up]; my father had died of lung cancer 25 years ago . (Patient 9) My only aim was to get back to normal , as if there were nothing wrong . (Patient 4) You have a lot of conflicting feelings; you wish this weren’t happening; you want to run away , but you say , “Where am I going to run to ? ” . (Patient 14)
Impact of medical communication . Several women comment that, when given the diagnosis, they dissociate because of the emotional impact and that they don’t listen to all the information that the medical professional is giving them.
I remember that she talked and talked , but I didn’t know what she was saying until she said , “Isabel , you’re going to be cured , okay ?”. (Patient 9)
During the diagnostic testing, the women are highly sensitive to the healthcare professionals’ words and gestures.
I looked at the face of the person who was doing the mammogram and that’s when I started to imagine the worst . (Patient 20) I say to them , “ But , is there a solution to this ? ” , and they say to me , “Don’t worry , I’m sure there is a solution . ” That “sure” is etched in my mind . (Patient 10)
Communication and managing their surroundings . After the diagnosis, the patients feel that they have to tell the people around them about their situation, especially those closest to them, the family. They all agree on how hard it is to share. Normally, the people it’s hardest to tell are their mother and their children. When they do, they try to put the most positive spin on it possible, in an attempt to keep them from worrying.
You no longer think only about yourself , you think , “Good grief , now I’ve got to tell my mother .” It’s hard . (Patient 16) I wanted to tell my kids the way I say things , always trying to look for the upside , and positive , although it was hard , but , anyway , in the end , it went well . When I finished , my husband told me , “You’ve convinced me that it’s no big deal .” (Patient 9) I told my son , “Son , don’t cry , your mom’s going to get over this , this is nothing .” (Patient 1)
During this period, the women contemplate how their situation will affect their surroundings and they try to organize it as much as possible.
I devoted myself to planning everything , to organizing what to do with my daughter , and to thinking about work , too , how I had left things at work . (Patient 4)
Surgery/cleaning out the cancer
Uncertainty and fear . The participants express that before going into surgery, they are told about the kind of procedure that will be done, but that, depending on what they find and the analysis, it may change. In light of this, they exhibit an enormous feeling of uncertainty and fear. In addition, many voice concern about how the surgery will go.
They tell you conservative surgery , but if we open up and see something we didn’t see on the tests , then everything could change . (Patient 10) Aside from the anesthesia , that I’m terrified of , you spend several hours in surgery and you don’t really know how things will go; when they clean it out , they analyze it , and you go into the operating room and you don’t know what can happen . (Patient 9)
Feeling of loss . Considering that the breast is associated with an intimate, feminine part [of their body], many women experience the operation as a loss. This loss is more acute if the operation is a mastectomy and there is no reconstruction at the same time. The loss also involves a loss of identity, compounded by the side effects of chemotherapy, such as hair loss. The interviewees who had undergone mastectomy say that following surgery, when the bandaging is removed and the scar is revealed, is one of the most critical moments, which is why they express difficulty in managing it and appreciate the caring assistance from the professionals.
It is identification with yourself , you know , it’s what you’ve seen in the mirror , what you think you’re like and , suddenly , you’re no longer like that; there’s an incredible personal crisis because you no longer recognize what you’re seeing . (Patient 11) I closed my eyes and I removed the bandaging and I didn’t dare look … with my eyes , I imagined the worst . (Patient 12)
Acceptance or demand for more aggressive intervention . The patients perceive the surgery as essential to recovering their health, which is why the process is widely accepted. Some patients who demand a more invasive intervention, normally a bilateral mastectomy, do so because that way, they feel safer with respect to a possible relapse, as well as more comfortable esthetically.
If they have to remove my breast , let them take it; what I want is to get better . (Patient 16) They say that I am in full remission , so they only removed the lump , but at first , I said that I wanted my whole [breast] removed ; then they assessed how to do it . (Patient 13) They told me that I had a genetic mutation and more possibilities of developing breast cancer and , since I felt such rejection toward my remaining breast , I decided to get rid of that one , too . (Patient 20)
Chemotherapy/loss of identity
The chemotherapy phase is one of the phases that affects the women’s lives the most, because of its physical impact and how long it lasts. No differences have been found in how they experience chemotherapy depending on whether it was neoadjuvant or adjuvant.
Negative impact of side effects . Chemotherapy is associated with many side effects that vary from one woman to another. Many indicate that they have suffered physical discomfort, such as fatigue, dysgeusia, pain, nausea and vomiting, mucositis, diarrhea, etc.
One day when I didn’t want to go to bed , I went to bed crying because I had the feeling that I wasn’t going to wake up . That day it was because I felt awful . (Patient 1)
Furthermore, all of the women suffer hair loss, which is one of the most-feared effects. Likewise, their body hair also falls out, especially on their face, and their weight fluctuates. All of these changes lead to a loss of identity that is experienced as taking away from their femininity. It must be remembered that oftentimes, chemotherapy is administered after surgery, further exacerbating this physical change. On top of all that, several women comment having to decide at the beginning of treatment whether to freeze their eggs or not; at that moment, many of them forfeit the possibility of becoming a mother or of becoming a mother again, which also adds to this loss of femininity.
Losing my hair was hard , but when it grew out again , I had an identity crisis . I didn’t recognize myself; people said I was really pretty like that , with my hair so short . I looked at myself in the mirror and I said that I’m not that woman , I can see that that woman is pretty , but it’s just that I don’t recognize myself . That’s not me or , it was like , I looked at myself and I didn’t recognize myself . That’s when I suffered a serious identity crisis , psychologically serious , but also serious because I sobbed because I looked at myself , but it wasn’t me . (Patient 6) Where’s that sexy lady , where is she ?, because you don’t feel good . I didn’t like myself at all . I was several sizes larger and I looked at myself and said , “What a monster . ” I didn’t feel good about myself . (Patient 1)
Many patients say that chemotherapy decreases their libido and dries up their mucous membranes, which is why they prefer not to have sex. For those who live as a couple, this situation can strain the relationship.
Sexually , I just didn’t feel like it , I wasn’t in the mood; not only did I not feel like it , my mucous membranes were dry and , what’s smore , I just couldn’t , I couldn’t , I felt bad for my husband , but he said , “Don’t worry .” (Patient 16)
Finally, some interviewees expressed a feeling of being poisoned by the treatment. These women tend to be highly focused on taking care of their body and have a very natural attitude toward life.
I had to really work my awareness that I was poisoning myself; at night I was at home and I thought that all that red liquid was circulating through my veins … I think I even had nightmares . (Patient 4)
Balance between caring for oneself and caring for others . The patients feel that it is time to take care of themselves, so they prioritize resting when they need it. Moreover, they worry about getting a haircut and, most of the times, they look for turbans and wigs. Some also learn how to put on make-up, which they rate as being very positive. On the other hand, those who have children or another person in their care, try to take care of them as much as they are able.
Around 11 : 00 , I no longer felt good , so I’d go to the armchair to rest and it’s like I had an angel , because I’d wake up a minute before I had to set the table and get lunch for my son who would be coming home from school . (Patient 1) While I was getting chemo , I went with the gadget and I told myself , “I’m going to teach you to apply make-up; for instance , your eyelashes are going to fall out . Make a line like this ” and at that moment when you look in the mirror , and we look like Fester in the Addams family . (Patient 13)
Vulnerability . The women experience great uncertainty and feelings of vulnerability the first times they receive chemotherapy, since they don’t know what side effects they will suffer.
With chemo , I started with a lot of fear and , later on , I became familiar with it little by little until the time comes when you go to the hospital like someone who’s going to pick up a bit of paper . (Patient 9)
In addition, those participants who join a social network or who are more closely tied to the hospital setting, know about the relapses and deaths of people around them diagnosed with breast cancer, which makes them feel highly vulnerable.
There are some people who leave the group because … it’s not like there are a lot of relapses and , geez , I think that it messes with your head . (Patient 13) We were almost always the same people at chemotherapy ; there was one guy who was really yellow who looked terrible and , there was one time when we stopped seeing him and another lady asked and the nurse said that he had died . (Patient 15)
At the same time, given the physical changes, especially those that have to do with body hair, many women feel observed when they leave home.
If I have to go out and take off my scarf because I’m hot or go straight out without any scarf on my head and whoever wants to look… let them ; I think that it’s up to us , the patients , to normalize the situation; unfortunately , there are more and more cases . (Patient 9)
Telling the kids . Since when the chemotherapy stage is going to entail many physical changes, the women look for ways to talk to their children about the treatment. Most of them comment that it is a complicated situation and all of them try to talk to their children in such a way as to protect them as much as possible.
I asked the nurse for help before I started chemotherapy to see if she had any pointers about how to talk about this with the kids and she recommended a story , but when I saw it , I didn’t like it … so , in the end , I decided to do it off the cuff . (Patient 10)
Radiotherapy/transition to normality
The “last” treatment . When the patient reaches radiotherapy, normally, they have already spent several months undergoing physically aggressive medical procedures, which is why they feel exhausted. There is a physical exhaustion resulting from the previous treatments and made worse by the radiation therapy. Furthermore, many women also report feeling emotionally drained by the entire process. However, this is generally accompanied by joy and relief because they feel that they are in the final stage of treatment.
Emotionally , it’s a marathon that has to end up at some point . (Patient 10) For me , radiotherapy was like a lull in the battle , with a winning mind-set . (Patient 4)
Comparison with chemotherapy . There is a widespread perception that radiotherapy has fewer side effects than chemotherapy, although later, when they receive it, several patients suffer discomfort, above all fatigue and dizziness. Several report that at this point, they are mentally worn out and just want to be done with the process, which is why they have less information than about chemotherapy.
I feel like radiotherapy is unknown , that you think it’s more “light ” and it turns out not to be so light . (Patient 13)
Follow-up care/the “new” day-to-day
Difficulty in getting back to normal . Once the patients are discharged, many feel that they need some time to recover, that it will be slow, in order to restore a more normalized pace of life. They are still working on their emotional and personal process.
When they tell you that you have cancer , they make it very clear : you have a goal; you have some months of chemo , some months of radio , and when you finish , you say , “And now , what do I do ?”. I say that because now I have to get back to my normal life , but I don’t feel normal . I still don’t feel cured , I’m not 100% . And you’re glad you’ve that you’ve finished it all and you’re alive , but at the same time , you say , “Gosh… this is very odd . ” It was a very strange feeling . (Patient 8)
Most patients report that their quality-of-life has diminished, due to the sequelae from the treatments. Lymphedema is one of the sequelae they name most often, although they also mention other symptoms, like digestive upset, weight issues, eye problems, scar pain, etc. The women who are on hormone therapy also suffer side effects, such as joint and muscle pain.
I have lymphedema and , although I have good mobility , I’m a little bit weak; when I go out for dinner , I generally order fish , because I can’t always cut meat well . (Patient 6)
Several interviewees also express difficulty in their affective-sexual relations. Many of them feel insecure because of all the physical changes; others have sequelae that hinder their relations, and still others are suffering symptoms of early menopause. This can cause problems in the couple and for those who don’t have a partner, suffer many complications when it comes to meeting other people.
I haven’t had sex with my husband for 2 years because , it’s also really complicated to get over; I’ve gone for pelvic physical therapy; I’ve used gels , but nothing works . (Patient 8) It’s taken me many months for me to have a relationship again; it’s been really hard because , even though everyone told me that I looked fine , I didn’t feel fine . My breast cancer had taken away all my attributes as a woman . (Patient 6)
Some women also experience difficulties when it comes to returning to work. Several state that they had been fired when they went back. They also report that when interviewing for a job, it’s complicated for them because they have to explain what happened and they mention the schedule of doctor’s visits that they have. Other women comment that they’ve been given early retirement or disability.
You go to the interview and if you tell them that you’ve had the disease , they look at you like you’re a weirdo . (Patient 13)
Breast reconstruction . How reconstruction is experienced, as well as its timing, are highly contingent upon they type of reconstruction. Each one has its pros and cons, but the opinions collected with respect to the type of reconstruction have been positive.
Although it took 18 months for the entire process to be over , I’m delighted with reconstruction with the expander . (Patient 16)
Some patients state that after the whole process, which has been long and complicated, they prefer not to undergo reconstruction immediately. In these cases, they report having felt a subtle pressure from the outside to undergo reconstruction.
Every time I went for my check-ups , they said , “You’re the only one left [who hasn’t undergone reconstruction]” and in the end , the truth is that I’m really happy because I think I look pretty . (Patient 12)
Check-ups and fear of relapse . Check-ups are one of the times that generate most worry and insecurity. The women remark that, starting a few days before and until they receive the results of the follow-up studies, they are more anxious about the possibility of relapse.
At every check-up my legs start shaking again and my stomach is in knots, although at my last one, everything turned out okay and I’m thrilled. (Patient 6) During the first stage , I did everything I had to do and I got over it , but it’s a lottery . You can do whatever you want , but it’s the luck of the draw and when you start going for check-ups , it’s like going to play Russian roulette . (Patient 8)
Maintenance hormone therapy . Hormone therapy is understood differently depending on age and on the major decision of whether or not to be a mother or to have another child. If the woman does not want to have more children, the treatment is accepted better. The patients who take it also report effects derived from menopause, for instance, joint pain or dry mucous membranes.
I did notice joint pain , but since I exercised , [I felt it] much less than my fellow women , although , for instance , when it comes to getting up from a chair , you get up like an old lady . (Patient 10)
Position of support . Several patients mention that, after discharge, they stay active on social media, they volunteer when they find out about someone or to participate in activities related to breast cancer, with the aim of being able to help other people who are in this situation.
It’s really good to meet other people who are going through the same thing , so , now that I’ve finished , I like it and I always help whenever I can , because I can share what was good for me . (Patient 13)
Relapse/starting over
Emotional impact . The diagnosis of a relapse is experienced much the same as the initial diagnosis. All of the women report fear, although they also state that they are more familiar with the processes. Other emotions emerge, such as why me, blame, disbelief, etc.
Since they had told me that it wasn’t going to happen again , I believed it , of course , I wanted to believe it and it totally surprised me; I couldn’t stop crying and crying . (Patient 17)
Telling the family again . Patients repeat that telling the family about it again, especially the children and parents, is tough and they try to minimize it in an attempt to protect them emotionally.
On the very same day that I had my mammogram , my mother says that she wants to come a see the kids . We’re in the park , when she arrives , I have to tell her that everything’s fine and when we get home , I tell her everything . My mother’s devastated again and I tell her not to worry , that everything is going to be fine . (Patient 16)
Thinking about whether something could have been done differently . Several women comment that, after their relapse, they think about whether the treatment was enough or there must have been something they could have done to avoid the relapse.
You get furious , because you say , “I wasn’t supposed to get sick , because if , 2 years ago when the first microcalcifications appeared I had had them removed , then I wouldn’t have metastasis , or maybe I would . (Patient 19)
Metastatic breast cancer/time-limited chronic
Re-interpreting the concept of metastasis . Most of the participants in this stage state that they have had to give new meaning to the word, “metastasis,” since their first perception was directly related to death. In this way, they come to understand that cancer can become chronic, although they now have to take medication and go to the hospital on a regular basis. Nevertheless, they know that their life expectancy may be a few years. The women who are involved in a group point out how hard it is to see their fellow member pass away.
What I now call my “ new normal” consists of lots of visits to the hospital and never going back to work . (Patient18)
They also state that at this stage, they do not identify with the disease generally known socially as “breast cancer”, where there is great emphasis placed on early detection and on their chances of being cured. This causes them to feel more isolated.
These pink ribbon campaigns hurt us because they tend to underscore that everything is going to turn out fine because breast cancer has a very high cure rate; there is huge lack of awareness . (Patient 20)
Physical and emotional discomfort . Most of the women in this stage report side effects from the treatments, although some comment that good quality-of-life can be preserved. On an emotional level, they say that they sometimes feel a certain agony due to not knowing how much longer the treatment will be effective. They live in a state of uncertainty that they try to cope with by focusing on their day-to-day and experience the good times deeply.
When I’m not in pain , I try not to even remember what I have and go out and have fun with my family and live . (Patient 20)
Several women who have children express with regret that they worry about their children enjoying them and remembering them when they were well. They are sad that they won’t be able to grow up in a normal family. Some also comment the impact this diagnosis is having on their partner.
What I don’t want is for them to carry this baggage of having a sick mother . (Patient 18)
A conflict with disability also appears, as many women report their desire to continue working, but feel that they can’t keep up with the pace of work. Additionally, several state that going through the medical board is a strenuous process, given that they look good physically.
It’s hard to deal with , I’m a non-practicing lawyer and I have degrees galore , but I worked the first year and I couldn’t continue . (Patient 21) Every year they call me again for the disability monitoring and they always threaten me . To be honest , the treatment doesn’t make me sick , but I don’t know how long it’s going to be like this . (Patient 19)
Social invisibility . The participants say that they do not have any physical signs of being ill, that they look fine, although they know and feel that inside, they are not well. They say that it is sometimes hard to manage socially, since on occasion, they feel misunderstood and disparaged.
I’m much sicker now , but people think or want to think that I’m fine . When I was doing chemo , it was like wearing a sign that said “cancer . ” (Patient 17)
This study describes the patient journey of women with breast cancer, specifying the different phases with the most relevant aspects of each, as well as the different cross-sectional features they report throughout the entire treatment process.
The results portray breast cancer as a process in which there is a striking feeling of loss of health and self-identity, changes in routines, personal and employment transformation, as well as emotional hardship during and after breast cancer treatment, aspects that are also reported in the literature [ 41 , 42 ]. Earlier studies state that experiencing cancer is highly stressful. It involves a major threat to life or physical integrity, in addition to mental health, interfering with the path, projects, and plans patients have for their life over the short, medium, and, on occasion, long term as well [ 6 ]. Along with reporting adverse physical and psychological impacts, patients also report positive ways in which they have grown psychologically or emotionally from the experience [ 7 , 42 ]. The diagnosis of breast cancer not only impacts the women individually, but also affects their surroundings. As reported in the literature, despite going through a very challenging time, the women struggle to put on a positive face and attempt to conserve the family’s well-being, specifically that of their children [ 7 ]. At the same time, the family is a fundamental source of support and usually provide indispensable support; however, it is not always effective, because family members do not fully understand the stresses involved in living with cancer [ 43 ]. Previous studies also reveal that for some women, their partners are one of their most significant supports; nonetheless, research also suggests that a cancer diagnosis predicts marital breakup more strongly for female survivors than males [ 44 ]. Our results reflect that the women frequently resort to other women in the same situation, possibly because they face significant unmet supportive care needs [ 30 ]. The need for social support may lead patients to seek social support groups consisting of people who are experiencing similar health crises, because such groups allow them to interact with those who best understand their suffering [ 43 ]. Another aspect that appears across the board is the relationship the participants have with the medical team. In this study, we have noted their trust in the medical team and acceptance of the treatments proposed without going into the clinical data of the disease and without needing to know the benefit provided by the treatment. Cancer patients are confronted with a potentially life-threatening [condition], feeling vulnerable, and need to rely heavily on their care providers, expecting the physician to act in their best interests [ 5 ]. Therefore, they need to have a close relationship, as well as comprehensive care [ 30 ]. Patients’ trust in a physician has been associated with a reduction of their fears and anxiety and [increased] satisfaction and adherence to treatment [ 5 , 30 ]. We believe that it would be important to provide patients with accurate information, so as to avoid misunderstandings (such as cancer being synonymous with death, regardless of stage) as several participants in this study have reported, which can lead them to believe that the risk of relapse with and without chemotherapy is much greater than the oncologists estimate [ 45 ]. We believe that in future studies that it would be worthwhile to examine the peculiarities of each kind of patient information with the aim of determining how to break it up and make it both comprehensible and tolerable to promote patients’ well-being.
A breast cancer diagnosis is generally unexpected and practically all patients suffer psychological distress, such as feelings of uncertainty, disbelief, hopelessness, vulnerability, anger, fear, anxiety, and sadness [ 46 , 47 ]. The literature has reported that many women experience peritraumatic distress or dissociation during the medical conversation in which they are given their diagnosis of cancer [ 48 ], which might account for the reactions of the respondents. Given that, when they receive their diagnosis, additional information is generally given to them, such as clinical aspects and preferred treatments. Repeating this information at subsequent appointments could contribute to improving communication with patient, since several participants stated that they found it hard to pay attention to the physician, given the emotional impact. Additionally, breast cancer patients tend to be diagnosed when they are relatively young, and often when they are in the middle furthering their career or raising children [ 12 ]. In spite of everything, the women try to put on as brave a face as they can and focus on maintaining their children’s well-being [ 7 ]. Telling children about their diagnosis is reportedly one of the biggest challenges; parents are usually unsure of how to tell them, because at the same time that they want it to be open and honest and cover their children’s developmental needs, they also want to protect them children [ 49 ].
Once diagnosed, breast cancer patients go through different treatments. The most salient experiences of these phases pertain to the impact of side effects on physical quality-of-life and psychological well-being, which is consistent with the literature [ 11 ]. Moreover, cancer therapy entails physical changes that affect their feminine identity, fertility, self-esteem, sexual functioning, and makes them more vulnerable [ 10 , 50 ]. Women described their inner self as being on an emotional rollercoaster with highs and lows throughout the various phases of treatment [ 7 ]. Given treatment side effects and sequelae, these women are more likely to experience physical symptoms and psychological disorders than patients with other kinds of tumors [ 51 ]. The side effects involve an acute sense of loss of health and quality-of-life, as well as identity and femininity. It would be interesting for future research to explore the therapies used in grief counseling with cancer patients, as understanding and exploring this perspective could comprise an additional clinical aid.
Once the women have completed their treatments, they gradually get back to normal and many contemplate returning to work. However, in line with our results, the literature reveals that even though they want to normalize their lives, female breast cancer survivors feel that they will never return to their baseline status [ 7 ]. A significant number of patients experience difficulties in physical, cognitive, and emotional functioning after their treatment, such as symptoms like lymphedema, fatigue, pain, sleep disorders, cancer-related cognitive impairment, emotional stress, symptoms of depression and anxiety, problems with relationships, reduced sexual identity, fertility problems, and fear of cancer relapse [ 13 , 14 ]. Furthermore, patients with hormone therapy suffer hot flashes, sweats, joint pain, weight gain, decreased libido, and low energy [ 52 ]. A sizeable number of these women also experience changes in employment status which can happen even 5–10 years following diagnosis [ 15 ]. Given that all these changes alter the structure of the woman’s everyday life, personalized care and treatment plans in cancer survivors are highlighted in the literature with extended specialized support being proposed that enables them to make a better psychosocially adjusted transition from treatment to follow-up [ 53 ] and advocating for the patient’s participation in all decisions that affect her during this period [ 54 ]. Further research is needed concerning how to structure the follow-up and support offered to these women during this stage so as to meet their needs and help them adjust to their new reality with the chronic sequelae caused by cancer and its treatment. On the other hand, the personal transformation of the initial stages of the journey are best seen during this phase. The literature shows that women who have had breast cancer report changes in their philosophy of life, such as embarking on a new life path, changing their priorities in life, as well as valuing life in general [ 42 ]. Most of the participants in our study place special emphasis on appreciating life, enjoying it more, and living each day to the fullest. Cancer survivors report being aware of how precarious life is, while also feeling the joy of being alive [ 55 ]. Similarly, they have been found to be more resilient and better able to repair their mood than healthy women [ 56 ].
About 5% of all patients with breast cancer are diagnosed when the disease is metastatic, whereas some 30% have suffered a relapse of an early breast cancer [ 17 ]. We saw that some women suffering a relapse after initial treatment with curative intent tend to wonder if the treatment was sufficient or if they should have done something more to prevent the relapse. Metastatic breast cancer is uncurable, which is why these women’s main psychosocial challenges are not the same as those who are diagnosed in early stages [ 18 ]. Faced with incurability, the women react with shock and fear of imminent death, but this anxiety diminishes once they begin treatment and learn that there are more treatment options [ 17 ]. During this phase, the interviewees reported impaired physical QoL and functioning, being hindered by pain, fatigue, or menopausal symptoms. Emotionally, they report suffering bouts of depression and anxiety, as well as fear because of the spread of their cancer. As for their relational QoL, their children’s welfare is their number one concern, especially for mothers of young children [ 17 , 57 ]. What’s more, these women felt isolated from society in general and, more specifically, from the non-advanced breast cancer community, inasmuch as they feel that nobody understands what they are going through [ 18 ]. A psychosocial approach is especially important in this phase to help these women to continuously adapt to the changes of their individual clinical situation and to the progression of the disease, thereby improving their coping.
Clinical implications
Having first-person information enables us to comprehend in detail the experiences of breast cancer patients, their situation, and emotional state, which favors holistic cancer care for health professionals.
Healthcare professionals should prepare women for a changed life situation, as well as to face prolonged, multimodal treatment (surgery, chemotherapy, hormone therapy, radiotherapy), and to confront physical and psychological sequelae, as well as the fear surrounding an uncertain prognosis. It is important to help them manage their expectations and fears and, to identify and address the issues and concerns that arise at different time points during treatment. The information and support offered should be adjusted to each woman’s individual needs, her life situation, her coping style, and the time and stage of their cancer. This more empathic, understanding outlook can also contribute to improving the physician-patient rapport, promoting communication, understanding, and shared decision-making.
Finally, a comprehensive understanding of the women’s psychosocial support endorses their belonging to groups of women with breast cancer, in which there is a relationship among equals. Further research is needed to specify the type needed so as to decrease both the impact of the death of women in the group, as well as the vast amount of information that they may end up obtaining, without needing it or requesting it.
Limitations
This study was performed with Spanish participants, which is why certain aspects cannot reflect the experiences of breast cancer patients from other countries, given the particularities of both the Spanish healthcare system and Spanish culture. Likewise, the data attained were specific to women with breast cancer, which can scarcely be extrapolated to individuals with other cancers. Moreover, the findings do not reflect men’s experiences with breast cancer and research with this group would enrich the field further. In addition, the age of our participants ranged from 34 to 61 years; hence the results should be interpreted for a middle-aged population and do not reflect the experiences of women diagnosed at very early or very old ages. Finally, we believe that there may be a bias regarding the women who agree to participate, as this group has probably accepted their condition more, as well as having worked on it more.
Despite these limitations, we hope that our findings can contribute to better understanding the experiences of women with breast cancer.
Acknowledgments
The authors are grateful to the investigators of the Neoetic study and the Bioetic Group of the Spanish Society of Medical Oncology (SEOM) for their contribution to this study. We would like to thank all the women who generously shared their experiences with us, the support of HealthyOnco ( www.healthyonco.com ), and Priscilla Chase Duran for editing and translating the manuscript.
Funding Statement
This work was funded by the Spanish Society of Medical Oncology (SEOM) in 2018. The sponsor of this research has not participated in the design of research, in writing the report, or in the decision to submit the article for publication.
Data Availability
From the kitchen to the laundry room, 5 home upgrades you didn't know you needed — from $8
- TODAY Plaza
- Share this —

- Watch Full Episodes
- Read With Jenna
- Inspirational
- Relationships
- TODAY Table
- Newsletters
- Start TODAY
- Shop TODAY Awards
- Citi Music Series
- Listen All Day
Follow today
More Brands
- On The Show

Jill Martin opens up after mastectomy: ‘I want to share this journey in real-time’
After an unexpected breast cancer diagnosis, six weeks later the TODAY contributor shares an update on what happens next.
What an earth-shattering six weeks. It feels like both yesterday and a lifetime since the last time I walked into Studio 1A. In the past six weeks, I learned I am positive for a BRCA2 mutation, had stage 2 breast cancer, then had a double mastectomy and learned that my life will soon look very different. Despite regular mammograms and sonograms, it wasn’t until genetic testing that I learned I have a gene mutation. A gene mutation that is the cause of my breast cancer.
Read more about Jill’s breast cancer diagnosis and her message about genetic testing here.
The surgery was three weeks ago. Honestly, physically, I am doing OK. (Emotionally is a different story; it's been a roller coaster.) My double mastectomy was successful. A week after my surgery, my 45-minute meeting with my oncologist, Dr. Joseph Sparano at Mount Sinai in New York City, started with the news that there is a good chance I am cancer-free, but I need further treatment to help ensure that.
The results of the tissue my medical team had biopsied had come back, and they believed they had gotten it all. I had an aggressive tumor removed, and my surgeon, Dr. Elisa Port, removed 18 lymph nodes, one of which was cancerous. They actually test the nodes in real time, so I found out the results of the biopsy when I woke up from the four-hour surgery. There is always a small possibility that cancerous cells could have escaped, so the rest of the meeting was dedicated to planning out my long road ahead, and what the doctor suggests I do to prevent this insidious disease from coming back. Honestly, it was a good thing my husband was with me and wrote everything down because, as you can imagine, it is a lot to take in.
I have to say this before I say anything else: I cannot tell you how helpful, inspiring, heartwarming, and life-changing it was to get all of your messages of strength and prayer after I shared the news of my breast cancer diagnosis. I received so many DMs, texts, emails, and calls. Strangers came up to me on the street saying, “Can I please add you to my prayer list?” or, “I am going to get tested because of your story. I didn’t realize how important genetic testing was on both the mother and the father’s side of the family.”
I am so touched, blown away and humbled. That really is what has helped me during this slow journey of healing. What I didn’t realize was almost everyone I have spoken with has either been through a version of this or watched someone go through it. I personally think it is easier to go through it than watch your loved one suffer; at least, that has been my experience with my family. It hurts me the most watching them watch me go through this. It shakes a family and a household. The past three weeks have felt like a lifetime.
I have gotten word from so many of you that you got tested for gene mutations and have gotten your results back. Most of you have received good news, and some of you have tested positive and are now deciding what to do. After going through this first part of my treatment, I understand it is such a personal choice because everyone’s circumstances, diagnosis and outlook are very different. But again, it should be your choice.
The most important message from me? Many dear friends, viewers and family have said, “I am afraid to have any kind of genetic testing, as I am afraid of what I will find out.” I totally understand that sentiment and reasoning. But let me be very clear: Any preventative measures you can take, although not easy, are easier than battling cancer. This entire process is life-changing, but adding cancer to the mix is a different kind of battle — one I do not wish on anyone.
I have shared the happy, the sad and now the scary. We will get through this together.
Also, even if you test positive for a gene mutation that means you are more likely to get cancer, it is not as if preventative surgery is your only option. You can choose to get scanned more often and tailor a plan with your doctor. It's all about doing what is right for you . No one can force you to do anything if you are not ready. Knowledge is power. Technology is power. I am writing this to ask that you please be your own best friend and advocate and educate yourself. No family should have to go through this unnecessarily.
This coming Wednesday, I will find out my exact next steps and treatment plan from Dr. Sparano. He will look at the findings from my Oncotype test, which is a test that determines the activity of a group of genes and how likely they are to respond to treatment. I do already know for sure I will need to have another surgery, preventatively, to remove my ovaries and fallopian tubes to decrease my risk of ovarian cancer. In my case, I will need a full hysterectomy, as I have had fibroid issues in the past. I will also need to take anti-hormonal drugs for 5 years. And I will most likely need chemotherapy because of the aggressiveness of the tumor. That is the part that hit me the hardest — the idea of chemo.
Dr. Port has been my rock throughout the last few weeks and has told me numerous times: “Whatever it is, you will get through this. Chemo is not a walk in the park, but it is not always what you picture. There are good days and harder days, and people work, exercise and do many, if not all, of their favorite activities throughout it." She has spoken to me about ways to save my hair and has suggested I have a wig made, “just in case.”
I am still in a state of shock, of course. Dr. Port told me that most people reconcile and process leading up to the surgery after being diagnosed. Yet, all of this happened so fast for me. There was not a lot of time to process upfront, and a lot of that is going on now after the fact. Cancer has knocked me down. It has. I used to jump out of bed every day to begin work, but now every day is a choice. Do I feel like staying under the covers and crying? Yes. Every day. But I did that when I first started recovery ... and little by little, like today, I am choosing to get up. I am choosing to fight. And I am choosing to use my strength and platform to do my best to crush cancer.
My two nurses, who work with the incredible Dr. Mark Sultan, who is doing my reconstruction, will forever be in my life: La Rae and Marilou were extraordinary. The first time I looked in the mirror after my mastectomy, La Rae said to me, “You are under construction.” It was the first time I laughed, and I realized I am allowed to cry, to mourn, to smile, to laugh. To be.
I will be on and off the air dealing with treatment for the next six months, but I thought it was important to share this journey with you in real time. I have shared the happy, the sad and now the scary. We will get through this together.
Thank you again for all your love and prayer.
For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Stage 2 breast cancer
Stage 2 breast cancer means that the cancer is either in the breast or in the nearby lymph nodes or both. It is an early stage breast cancer.
The stage of a cancer tells you how big it is and how far it has spread. It helps your doctor decide the best treatment for you. There are different systems used in the UK to stage breast cancer. Stage 2 is part of the number staging system. Doctors may also use the TNM staging system.
Staging for breast cancer is very complex. Many different factors are considered before doctors can confirm your final stage. Speak to your doctor or breast cancer nurse specialist if you have any questions about your staging.
Stage 2 can be divided into 2A and 2B. Below is a simplified description of stage 2A and 2B breast cancer.
Stage 2A means one of the following:

- the cancer is larger than 2 cm but not larger than 5 cm and there is no cancer in the lymph nodes
Stage 2B means one of the following
- the cancer is larger than 2 cm but not larger than 5 cm and there are small areas of cancer cells in the lymph nodes
- the cancer is larger than 2 cm but not larger than 5 cm and the cancer has spread to 1 to 3 lymph nodes in the armpit or to the lymph nodes near the breastbone
- the cancer is larger than 5cm and hasn't spread to the lymph nodes
The TNM staging system stands for Tumour, Node, Metastasis.
- T describes the size of the tumour (cancer)
- N describes whether there are any cancer cells in the lymph nodes
- M describes whether the cancer has spread to a different part of the body
In the TNM staging system, stage 2A breast cancer is the same as:
Stage 2B is the same as:
- Read more about the TNM staging system for breast cancer
The number staging helps your doctor decide which treatment you need. Treatment also depends on:
- the type of cells the cancer started in
- whether your cancer cells have receptors for particular cancer drugs
- the grade of the cancer
- other health conditions you may have
Your doctor will take many different factors into account when deciding which treatment is best for you.
Surgery is usually one of the main treatments for stage 2 breast cancer. You may also have drug treatments such as chemotherapy and hormone therapy as a first treatment. This is called neo adjuvant treatment. You then have surgery.
Your surgeon might remove the cancer and a border of normal breast tissue. This is called breast conserving surgery or a wide local excision. After breast conserving surgery, you might have radiotherapy to the rest of the breast.
Or you might have the whole breast removed. This is called a mastectomy. You can then choose to have a new breast made (breast reconstruction). You might also have radiotherapy to the chest wall after having a mastectomy.
- Find out more about surgery for breast cancer
Checking the lymph nodes
Before your surgery, you have an ultrasound scan to check the lymph nodes in the armpit (axilla). This is to see if they contain cancer cells. If breast cancer spreads, it usually first spreads to the lymph nodes close to the breast.
Depending on the results of your scan you might have:
- surgery to remove the lymph nodes under your arm
- Find out more about surgery to remove the lymph nodes
Other treatments you might have
You usually have other treatments too. These include:
- hormone therapy
- chemotherapy
- targeted cancer drugs
- drugs that strengthen the bones called bisphosphonates
- Read about the treatment options for breast cancer
Other stages of breast cancer
- TNM staging
Related links
Breast cancer treatment.
Treatment for breast cancer depends on a number of factors. Find out about breast cancer treatments, where and how you have them, and how to cope with possible side effects.
Tests for breast cancer
You have a number of tests to check for breast cancer. This includes a breast examination, a mammogram, a biopsy and scans.
TNM staging for breast cancer
The TNM system is a way of staging breast cancer. TNM stands for Tumour, Node, Metastasis.
Surgery for breast cancer
Most people begin their breast cancer treatment with surgery. Find out about the different types of surgery for breast cancer, how to prepare for your operation, and how to recover well.
Living with breast cancer
Get practical, physical and emotional support to help you cope with a diagnosis of breast cancer, and life during and after treatment.
Breast cancer main page
Find out about breast cancer, including symptoms, diagnosis, treatment, survival, and how to cope with the effects on your life and relationships.
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Find a clinical trial
Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
For Your Patients: Understanding Early-Stage Breast Cancer
— it is generally more treatable and has a better prognosis.
by Shalmali Pal , Contributing Editor, MedPage Today Reviewed By Eleonora Teplinsky, MD, Head of Breast Medical Oncology at Valley Health System in Paramus, New Jersey, Clinical Assistant Professor at Icahn School of Medicine at Mount Sinai in New York City, and host of the “Interlude: Cancer Stories with Dr. Teplinsky” podcast.

"Medical Journeys" is a set of clinical resources reviewed by physicians, meant for the medical team as well as the patients they serve. Each episode of this journey through a disease state contains both a physician guide and a downloadable/printable patient resource. "Medical Journeys" chart a path each step of the way for physicians and patients and provide continual resources and support, as the caregiver team navigates the course of a disease.
After a breast cancer diagnosis has been made, your physicians will need to determine the cancer's status, whether it has spread, and, if so, how far. This process is called staging and helps to determine the prognosis and the best treatment. Early-stage breast cancer is generally more treatable and is associated with a better prognosis compared with more advanced stages.
Treatment options for early-stage breast cancer include surgery (lumpectomy or mastectomy); radiation therapy; systemic therapy (chemotherapy, endocrine (hormone) therapy, anti HER2 therapy, and/or immunotherapy). Systemic therapy options depend on the specific characteristics of the tumor and your overall health and medical history.
Breast cancer staging is determined by a combination of factors including tumor size, lymph node involvement, grade, presence or absence of metastasis, and presence or absence of estrogen receptor (ER), progesterone receptor (PR), and HER2-neu status.
Managing early-stage breast cancer is a group effort that includes your medical oncologist, surgeon, radiation oncologist, primary care physician, and other healthcare professionals -- and most importantly, you.
Diagnosis/Staging
Early-stage invasive breast cancer refers to disease that has been detected at an early point in its development and has not spread beyond the breast and nearby lymph nodes. The disease is generally divided into stage 0 and stage 1 per the American Joint Committee on Cancer (AJCC) staging system.
AJCC staging was originally based on anatomy alone, using primary tumor size (T), nodal involvement (N), and the presence or absence of metastasis (M) (TNM staging). The 2017 8th edition of staging, however, presented a new prognostic staging system for breast cancer which in addition to TNM staging also includes tumor grade and estrogen receptor (ER), progesterone receptor (PR), and HER2/neu status.
Stage 1 is characterized by the presence of small tumors or cancerous cells that have begun to invade nearby breast tissue but have not yet spread to lymph nodes outside the breast or other areas of the body. Stage 1 has two substages:
- Stage 1A: Small primary tumor up to 2 cm in diameter, with no evidence of lymph node involvement or distant metastasis (T1, N0, M0)
- Stage 1B: Small primary tumor (<2 cm) located in breast tissue (T1, N0, M0)
There are other types of benign but high-risk breast conditions: atypical ductal hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ, which increase the risk of in situ and/or invasive carcinoma in either breast.
Early-stage breast cancers are often treated with breast-conservation therapy with surgical lumpectomy to remove primarily just the tumor.
Lumpectomy is typically followed by radiation therapy to eliminate any microscopic residual disease. Radiation is sometimes omitted in elderly patients with low-risk disease. The sentinel lymph node (defined as the first lymph node where cancer cells are more likely to spread from the breast), is often removed during the same procedure as the lumpectomy to identify lymph node involvement. Additional lymph nodes may be removed depending on the results of the sentinel lymph node biopsy.
Not all patients are candidates for breast-conservation therapy and in some cases, a mastectomy may be recommended. In a simple or total mastectomy, the entire breast is removed. This surgery includes the removal of the nipple, areola, fascia (covering) of the pectoralis major muscle (main chest muscle), and skin. In some cases, sentinel lymph nodes may also be removed.
A modified radical mastectomy combines a simple mastectomy with the removal of the lymph nodes under the arm, which is called an axillary lymph node dissection. A radical mastectomy is much more extensive with removal of the chest wall muscles and is rarely done. Other options are skin-sparing mastectomy and nipple-sparing mastectomy.
Your surgeon will discuss the advantages and disadvantages of each procedure with you, as well as breast reconstruction options you may wish to consider. You will meet with a plastic surgeon as well to discuss breast reconstruction.
Radiation Therapy
Radiation therapy after your surgical procedure may be performed to target any remaining cancer cells and reduce the risk of recurrence. For most people, this will take place over 3 to 5-6 weeks, although in some cases, it may be completed in a single week.
Your radiation oncologist will help you understand the details of the treatment plan designed for you and its potential side effects.
Systemic Therapy
Systemic therapy is designed to target and eliminate potential cancer cells throughout the entire body. The goal of this therapy is to reduce the risk of breast cancer recurrence as well as improve your long-term survival by destroying micrometastases that might not be detectable through imaging or other means.
Depending on the tumor's characteristics, systemic therapy is administered before surgery (called neoadjuvant treatment) or after surgery (adjuvant treatment). The main types of systemic therapy used in early-stage breast cancer are chemotherapy, endocrine (hormone) therapy, anti-HER2 therapy, and immunotherapy.
Neoadjuvant therapy offers an opportunity to evaluate tumor response and adjust therapy in the adjuvant setting if necessary.
A chemotherapy regimen is typically given in cycles, with rest periods in between to allow the body to recover.
Hormone therapy, also called endocrine therapy, is recommended if you have hormone-receptor positive breast cancer. Options for endocrine therapy include the selective estrogen receptor modulator tamoxifen, and aromatase inhibitors. Tamoxifen works by attaching to estrogen receptors on cancer cells, blocking estrogen from binding to these receptors, and preventing the estrogen-driven growth of cancer cells.
Hormone therapy is typically prescribed for 5-10 years.
Your oncologist will go over treatment options, duration, and potential side effects in detail.
Targeted Therapies
Different types of drugs target the HER2 protein.
Monoclonal antibodies are manufactured versions of immune system proteins, which are designed to attach to the HER2 protein on cancer cells and stop it from growing. Trastuzumab (Herceptin) and pertuzumab (Perjeta) are two monoclonal antibodies that are used in early-stage disease.
Other targeted therapies that may be possible in early-stage breast cancer are antibody-drug conjugates (ADCs) and kinase inhibitors. ADCs are linked to a chemotherapy drug, and kinase inhibitors block proteins that send signals to cancer cells, such as signals for it to grow.
Immunotherapy aims to stimulate the body's immune system to recognize and attack cancer cells. At this time, outside of a clinical trial, immunotherapy use for early-stage breast cancer is limited to stage II or III triple-negative breast cancer. The currently approved immunotherapy for early-stage breast cancer is pembrolizumab (Keytruda), which is a checkpoint inhibitor.
Treatment Side Effects
- Side effects from surgery can include bleeding and infection at the surgery site; pain, tenderness, or swelling at the surgical site; and limited arm/shoulder movement.
- Side effects from radiation therapy can include swelling, skin changes in the breast, and fatigue.
- Side effects from chemotherapy can include nausea, fatigue, hair loss, and skin conditions.
- Side effects from hormone therapy can include weight changes, hot flashes and night sweats, fatigue, and gastrointestinal symptoms.
- Side effects from targeted therapies can include diarrhea, severe fatigue, and more serious health issues such as heart damage.
The extent and degree of side effects from all treatments will vary by person and by the specific types of treatments used. Always inform members of your healthcare team if you have concerns about any symptoms or complications following any treatment.
Follow-Up and Surveillance
Your medical team will schedule regular follow-up appointments once you have completed active therapy. These visits are a crucial part of managing your health, monitoring your progress, and detecting any potential recurrence as early as possible.
Lifestyle, Self-Care, Support
Engaging in regular physical activity, eating a balanced diet, limiting alcohol use, and getting adequate rest, will play a significant and positive role in managing early-stage breast cancer.
Being diagnosed with breast cancer is an emotional challenge. Support groups of others who are managing their breast cancer are available, both locally and online, and will provide emotional and practical support.
Individual counseling may also be an option you may wish to explore to extend your support network.
Read previous installments in this series:
Part 1: For Your Patients: Breast Cancer Basics
Part 2: For Your Patients: The Crucial Role of the Biopsy in Breast Cancer
![stage 2 breast cancer journey author['full_name']](https://clf1.medpagetoday.com/media/images/author/spal_headshot_188.jpg)
Shalmali Pal is a medical editor and writer based in Tucson, Arizona. She serves as the weekend editor at MedPage Today, and contributes to the ASCO and IDSA Reading Rooms.
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
Stage II Breast Cancer
- Melanie Royce, MD, PhD
- Shari B. Goldfarb, MD
This management guide covers the treatment of stage II breast cancer, malignancies with primary tumors > 2 cm that involve ipsilateral axillary lymph nodes and tumors ≤ 5 cm without nodal involvement.
This chapter focuses on the treatment of stage II breast cancer, which encompasses malignancies with primary tumors > 2 cm in their greatest dimension that involve ipsilateral axillary lymph nodes as well as tumors up to 5 cm without nodal involvement.
Stage II breast cancer is further subdivided into stages IIA and IIB. Patients classified as having stage IIA breast cancer include those with T0-1, N1, and T2, N0 disease. Stage IIB breast cancer includes patients with T2, N1, and T3, N0 disease. Therefore, this patient population is more heterogeneous than are the populations with stages 0 and I disease. The pretreatment evaluation and type of treatment offered to patients with stage II breast cancer are based on tumor size, nodal status, status of receptors for estrogen and human epidermal growth factor receptor 2 (HER2, HER2/ neu ) and Oncotype DX recurrence score.
Surgical and Radiation Treatment
Multiple studies have demonstrated that patients with stage II breast cancer who are treated with either breast-conservation therapy (lumpectomy and radiation therapy) or modified radical mastectomy have similar disease-free and overall survival rates.
Breast-conservation therapy
The optimal extent of local surgery has yet to be determined and, in the literature, has ranged from excisional biopsy to quadrantectomy. A consensus statement issued by the National Cancer Institute (NCI) recommended that the breast cancer be completely excised with negative surgical margins and that a level I-II axillary lymph node dissection be performed. Patients should subsequently be treated with adjuvant breast irradiation.
Patients with tumors > 4 to 5 cm may not be optimal candidates for breast conservation due to the risk of significant residual tumor burden and the potential for a poor cosmetic result following lumpectomy (or partial mastectomy). Neoadjuvant chemotherapy, typically used for locally advanced breast cancer, is increasingly used in earlier stage, operable breast cancers to reduce the size of the primary tumor and allow for breast-conserving therapy.
In a study of more than 300 patients treated with neoadjuvant chemotherapy at the MD Anderson Cancer Center, promising results were reported. At a median follow-up of 60 months, the 5-year actuarial rates of ipsilateral breast tumor recurrence (IBTR)-free and locoregional recurrence–free survival were 95% and 91%, respectively. The authors concluded that breast-conservation therapy after neoadjuvant chemotherapy results in acceptably low rates of recurrence-free survival in appropriately selected patients, even those with T3 or T4 disease. Advanced nodal involvement at diagnosis, residual tumor larger than 2 cm, multifocal residual disease, and lymphovascular space invasion predict higher rates of recurrence.
In some patients, preoperative chemotherapy produces a sufficient reduction in tumor size that allows patients to receive breast-conserving therapy. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 trial showed that preoperative doxorubicin-based chemotherapy decreases tumor size by > 50% in approximately 90% of operable breast cancers, resulting in a greater frequency of lumpectomy.
In a subsequent trial, NSABP B-27, women with invasive breast cancer were randomized to receive 4 cycles of preoperative AC (Adriamycin [doxorubicin] and cyclophosphamide) chemotherapy followed by surgery, or 4 cycles of preoperative AC followed by 4 cycles of docetaxel (Taxotere) then surgery, or 4 cycles of preoperative AC followed by surgery then by 4 cycles of postoperative docetaxel. A higher rate of complete pathologic response was seen at surgery in patients treated with AC followed by docetaxel vs AC alone. There were no significant differences in disease-free and overall survival between the treatment groups. However, those with a complete pathologic response in the breast had significant improvements in disease-free survival (hazard ratio [HR] = 0.45; P < .001) and overall survival (HR = 0.33; P < .001) compared with those who had residual disease after preoperative chemotherapy. Because preoperative chemotherapy does not have a negative impact on survival, the preoperative approach is a reasonable option and has gained favor among many patients.
Preoperative chemotherapy had an ability to convert patients requiring mastectomy to candidates for breast-conserving surgery. However, there was an increase in local recurrence in the “converted” group vs those deemed eligible initially for breast-conserving surgery.
Patients undergoing sentinel lymph node biopsy. A prospective study designed to determine the survival impact of micrometastases in the sentinel nodes of patients with invasive breast cancer included 790 patients who underwent sentinel node biopsy. The investigators found no significant difference in 8-year disease-free or overall survival among patients with micrometastatic tumor deposits in sentinel nodes defined as being pN0(i+) or pN1mic when compared with patients having negative sentinel nodes. The true significance of these micrometastatic deposits is still unclear, but it may help to better define groups of patients who should receive maximum adjuvant medical or surgical therapy or who could avoid additional therapy.
Although helpful in prognosis and treatment planning, completion axillary dissection after a positive sentinel node biopsy may not be required for small tumors and in the absence of lymphovascular invasion. Factors affecting the rate of positive nonsentinel nodes and the necessity of axillary dissection following a positive sentinel lymph node resection were evaluated in NSABP B-32. Women with operable invasive breast cancer and clinically negative nodes were randomized to undergo sentinel node resection with immediate conventional axillary dissection (group 1) or without axillary dissection (group 2). Patients in group 2 who had positive sentinel nodes underwent axillary dissection. Data from 1,166 patients with positive sentinel nodes that were available for multivariate analysis (595 from group 1; 571 from group 2) indicated that a significantly higher percentage of patients in group 2 had positive nonsentinel nodes than did those in group 1 (41.5% vs 35.5%; P = .032). Clinical tumor size was a significant predictor for positive nonsentinel nodes ( P = .001). Percentages of patients having positive nonsentinel nodes were significantly increased by the number of positive sentinel nodes, and lymphovascular invasion was a significant predictor for positive nonsentinel nodes. The percentages of patients with positive nonsentinel nodes significantly decreased with increases in the number of hot spots identified and the number of sentinel nodes removed.
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial is a randomized, multicenter trial designed to determine the effects of completion axillary node dissection on survival of patients with clinical T1 or T2 N0 M0 breast cancer who have one to two positive sentinel nodes. Patients were treated with lumpectomy with whole breast irradiation and adjuvant systemic therapy. Clinical and tumor characteristics were similar between a group of 445 patients randomized to axillary lymph node dissection (ALND) and a group of 446 patients randomized to sentinel lymph node dissection (SLND) alone. At a median follow-up of 6.3 years, locoregional recurrences were uncommon, occurring in 3.1% of ALND and 1.6% of SLND patients ( P = .11). Only age (≤ 50 years) and higher Bloom-Richardson scores were associated with locoregional recurrence by multivariate analysis. Number of positive sentinel nodes, size of the sentinel node metastasis, and number of lymph nodes removed were not associated with locoregional recurrence. The omission of ALND in this group of patients did not result in inferior survival. The 5-year overall survival was 91.8% (95% confidence interval [CI], 89.1–94.5) with ALND and 92.5% (95% CI, 90–95.1) with SLND alone; 5-year disease-free survival was 82.2% (95% CI, 78.3–86.3) with ALND and 83.9% (95% CI, 80.2–87.9) with SLND alone. A drawback of this study is its early closure and accrual of less than one-half of the targeted enrollment population; nonetheless, its findings have immediate implications for clinical practice. It is important to note that the Z0011 trial did not include patients undergoing mastectomy, lumpectomy without radiotherapy, partial-breast irradiation, or neoadjuvant therapy. In those patients, ALND remains standard practice when SLND identifies a positive SLN.
The timing of sentinel node biopsy in patients undergoing preoperative chemotherapy is controversial. Preoperative chemotherapy can sterilize the axillary nodes and lead to errors in determining nodal involvement. ACOSOG 1071 was designed to determine the application of SLN surgery for staging the axilla following chemotherapy for women who initially had node-positive cN1 breast cancer. The primary objective of the trial was to determine if the false-negative rate (FNR) for SLN surgery with two or more LNs examined following chemotherapy in women initially presenting with biopsy-proven cN1 breast cancer was greater than 10%. A total of 756 women were enrolled, with 649 patients undergoing neoadjuvant chemotherapy followed by both SLN surgery and ALND. Of these 649, a total of 525 patients had two or more SLNs removed. In 39 patients, cancer was not identified in the SLNs but was found in lymph nodes obtained with ALND, resulting in an FNR of 12.6% (90% Bayesian credible interval, 9.85% to 16.05%). Their findings suggest that surgeons cannot reliably detect by SLN procedures all axillary lymph node metastases in patients with cN1 breast cancer following chemotherapy. The authors further concluded that for SLND to become an alternative to ALND in this patient population, changes in approach and patient selection that result in greater sensitivity would be necessary.
Radiation therapy after breast-conserving surgery
For patients with stages I and II breast cancer, radiation therapy following lumpectomy remains an acceptable standard of care. Randomized trials and single-institution experiences have consistently demonstrated a significant reduction in local relapse rates for radiotherapy following breast-conserving surgery. Furthermore, small but significant differences in distant metastasis and disease-free survival have been observed in randomized trials comparing lumpectomy alone with lumpectomy and radiation therapy for patients with invasive breast cancer.
Based on the results of a number of retrospective, single-institution experiences as well as several prospective, randomized clinical trials, breast-conserving surgery followed by radiation therapy to the intact breast is now considered standard treatment for the majority of patients with stage II invasive breast cancer.
An update from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) metaanalysis focused on 10,801 women enrolled in 17 randomized trials of radiotherapy vs no radiotherapy after breast-conserving surgery and relates the absolute reduction in 15-year risk of breast cancer death to the absolute reduction in 10-year recurrence risk. Overall, radiotherapy reduced the 10-year risk of any first recurrence from 35% to 19.3% ( P < .001) and reduced the 15-year risk of breast cancer death from 25.2% to 21.4% ( P < .001). In women with pN+ disease (n = 1,050), radiotherapy reduced the 10-year recurrence risk from 63.7% to 42.5% ( P < .001) and the 15-year risk of breast cancer death from 51.3% to 42.8% ( P = .01).
Radiation dose and protocol. Radiation dose to the intact breast follows the same guidelines used in patients with stages 0 and I disease, described in the previous chapter.
Regional nodal irradiation (RNI). For patients who undergo ALND and are found to have negative lymph nodes, RNI may be employed if the patient has high risk factors such as triple-negative disease, an undissected axilla, or inner/medial quadrant tumors. For patients with positive lymph nodes, radiation therapy to the supraclavicular fossa and/or internal mammary chain may be considered on an individualized basis.
RNI should be administered using careful treatment-planning techniques to minimize the dose delivered to the underlying heart and lungs. Prophylactic nodal irradiation to doses of 45 to 50 Gy results in a high rate of regional nodal control and may improve disease-free survival in subsets of patients.
The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) MA20 trial evaluated addition of RNI to whole breast irradiation (WBI) following breast-conserving surgery. Whelan et al reported that women with high-risk node-negative or node-positive breast cancer treated with breast-conserving surgery and adjuvant chemotherapy and/or endocrine therapy were randomized to WBI (50 Gy in 25 fractions +/− boost irradiation) or WBI plus RNI (with RNI at 45 Gy, in 25 fractions) to the internal mammary, supraclavicular, and high axillary lymph nodes. A total of 1,832 women were randomly assigned to WBI + RNI (n = 916) or WBI (n = 916). Median follow-up was 62 months. In the study population, 85% of the women had one to three positive nodes, 91% received adjuvant chemotherapy, and 71% got adjuvant endocrine therapy. Compared with WBI alone, WBI + RNI was associated with an improvement in isolated locoregional disease – free survival (HR = .59; P = .02; 5-year risk 94.5% vs 96.8%, respectively), distant disease – free survival (HR = .64; P = .002; 5-year risk 87% vs 92.4%, respectively), disease-free survival (HR = .68; P = .003; 5-year risk 84% vs 89.7%, respectively), and overall survival (HR = .76; P = .07; 5-year risk 90.7% vs 92.3%, respectively). WBI + RNI in comparison to WBI was associated with an increase in grade 2 or greater pneumonitis (1.3% and 0.2% respectively; P = .01) and lymphedema (7.3% and 4.1% respectively; P = .004).
Radiation therapy after mastectomy
Available data suggest that in patients with positive postmastectomy margins, chest wall fixation, primary tumors > 5 cm, or involvement of four or more lymph nodes at the time of mastectomy, the risk of locoregional failure remains significantly high enough for postmastectomy radiation therapy to be considered.
Several prospective, randomized trials have evaluated the role of postmastectomy radiotherapy in addition to chemotherapy. Most of these trials have been limited to patients with pathologic stage II disease or with T3 or T4 primary lesions. All of these trials have shown an improvement in locoregional control with the addition of adjuvant irradiation, and several recent trials have demonstrated a disease-free and overall survival advantage in selected patients. Clinical practice guidelines developed by the American Society of Clinical Oncology (ASCO) support routine use of postmastectomy radiation therapy for women with stage III or T3 disease or those who have four or more involved axillary lymph nodes.
Most ongoing trials evaluating dose-intensive chemotherapy routinely include postmastectomy radiation therapy to the chest wall and/or regional lymph nodes to minimize locoregional recurrence.
Current recommendations. There is no clearly defined role for postmastectomy irradiation in patients with small (T1 or T2) primary tumors and negative nodes. For patients with one to three positive nodes, postmastectomy radiation therapy may be considered to lower the rate of local relapse and improve disease-free survival, although the benefit is less than that of patients with four or more positive nodes. However, based on the NCIC-CTG MA20 trial, addition of RNI in node-positive patients is beneficial in terms of locoregional control and disease-free survival. For patients with four or more positive lymph nodes, with or without a large primary tumor, postmastectomy radiation therapy should be considered to lower the rate of local relapse and improve disease-free survival. For patients who are younger, or who have T1 or T2 tumors, one to three positive nodes, poorly differentiated subtypes, or lymphovascular invasion, postmastectomy radiation therapy may have a benefit with respect to disease-free and overall survival. However, controversies and uncertainties regarding this issue remain, and individualized decision-making based on the patient’s overall condition and specific risk factors is reasonable.
The EBCTCG conducted a meta-analysis of 8,135 women randomly assigned to treatment groups during 1964 through 1986 in 22 trials of radiotherapy to the chest wall and regional lymph nodes after mastectomy and axillary surgery vs the same surgery but no radiotherapy. In women who underwent axillary dissection and had no positive nodes (n = 700), radiotherapy did not affect locoregional recurrence or breast cancer mortality. In women who underwent axillary dissection and had one to three positive nodes (n = 1,314), radiotherapy reduced locoregional recurrence (2 P < .00001), overall recurrence (2 P = .00006), and breast cancer mortality (2 P = .01), even in the setting of chemotherapy. Notably, the unirradiated women in these trials with one to three positive nodes had an absolute 10-year risk of overall recurrence of 45.7%, which was reduced to 34.2% by their radiotherapy, so their absolute gain was 11.5%. For 1,772 women with axillary dissection and four or more positive nodes, radiotherapy reduced locoregional recurrence (2 P < .00001), overall recurrence (2 P = .0003), and breast cancer mortality (relative risk [RR] = 0·87; 95% CI, 0.77–0.99; 2 P = .04).
Minimizing pulmonary and cardiac toxicities. Early trials employing postmastectomy radiation therapy showed that the modest improvement in breast cancer mortality was offset by an excess risk of cardiovascular deaths, presumably due to the radiation treatment techniques used, that resulted in delivery of relatively high radiation doses to the heart. Recent trials employing more modern radiation therapy techniques have not demonstrated an excess of cardiac morbidity and, hence, have shown a slight improvement in overall survival due to a decrease in breast cancer deaths. Thus, in any patient being considered for postmastectomy radiation therapy, efforts should be made to treat the areas at risk while minimizing the dose to the underlying heart and lungs.
Radiation dose and protocol. The available literature suggests that doses of 4,500 to 5,000 cGy should be sufficient to control subclinical microscopic disease in the postmastectomy setting. Electron-beam boosts to areas of positive margins and/or gross residual disease reaching doses of about 6,000 cGy and delivered to sites of gross disease, may be considered. At present, in the United States, it is not standard of care to consider hypofractionated regimens in the setting of postmastectomy radiation therapy.
In patients who have undergone axillary lymph node dissection, even in those with multiple positive nodes, treatment of the axillae does not appear to be necessary in the absence of gross residual disease. Treatment of the supraclavicular and/or internal mammary chain should employ techniques and field arrangements that minimize overlap between adjacent fields and decrease the dose to underlying cardiac and pulmonary structures. When treating patients who underwent neoadjuvant chemotherapy who only underwent SLN surgery, care should be taken to comprehensively treat the regional lymph nodes based on the NCIC MA.20 and EORTC trials.
There are two prospective cooperative group trials that will help determine the role of the optimal local therapy after neoadjuvant chemotherapy. The Alliance for Clinical Trials in Oncology cooperative group (formerly known as the American College of Surgeons Oncology Group, Cancer and Leukemia Group B, and North Central Cancer Treatment Group) trial A011202 examines the role of local therapy in the management of the axilla in patients with residual node-positive disease after neoadjuvant chemotherapy. Patients with cT1-3 N1 disease treated with neoadjuvant chemotherapy will be enrolled, and patients with positive sentinel nodes will be randomly assigned to axillary radiation or completion axillary lymph node dissection. All patients will receive radiation to the breast or chest wall and to the undissected supraclavicular and level III axillary nodes. A companion study by the NRG Oncology group (formerly known as the National Surgical Adjuvant Breast and Bowel Project, the Radiation Therapy Oncology Group, and the Gynecologic Oncology Group) trial 9353 examines the role of local therapy in the management of the axilla in patients who convert to node-negative after neoadjuvant chemotherapy. Patients with cT1-3 N1 disease treated with neoadjuvant chemotherapy will be enrolled and those with negative sentinel nodes will be randomly assigned to comprehensive nodal irradiation or no nodal irradiation. All patients will receive radiation to the breast or chest wall.
Medical Treatment
Medical management of local disease depends on clinical and pathologic staging. Systemic therapy is indicated only for invasive (infiltrating) breast cancers.
A discussion of the sequencing of chemotherapy and irradiation and hormonal therapy with irradiation is provided in the previous chapter.
Treatment regimens
Systemic adjuvant therapy has been shown to decrease the risk of recurrence and in some cases also the risk of death. Systemic therapy may be divided into chemotherapy and endocrine (hormonal) therapy. Chemotherapy often involves use of combination regimens, given for 4 to 8 cycles. It is most often delivered after primary surgery for breast cancer and before radiation therapy for those who are candidates for irradiation.
A study comparing two decision aids found that the 21-gene assay (Oncotype DX) was more accurate than were the classic clinicopathologic features and therapy utilized in Adjuvant! Online in predicting recurrence among 465 women with hormone receptor–positive operable breast cancer and zero to three positive axillary nodes who were treated with chemohormonal therapy. Recurrence score highly significantly predicted recurrence of both node-negative and node-positive disease ( P < .001) when adjusted for other clinical variables. Recurrence score also was more accurate than were clinical variables at predicting recurrence when integrated by an algorithm modeled after Adjuvant! Online that was adjusted to 5-year outcomes. The 5-year recurrence rate was 5% or less for the 46% of patients with a low recurrence score (< 18). The investigators concluded that the 21-gene assay may be used to select low-risk vs high-risk patients for specific chemotherapy regimens and clinical trials.
A multicenter study found that the recurrence score assay impacts medical oncologists’ adjuvant treatment recommendations and patient treatment choice. The largest change due to recurrence score assay results was conversion from the medical oncologist’s pretest recommendation for chemotherapy plus hormonal therapy to post-test recommendation for hormone therapy alone, and the recurrence score results often increased medical oncologists’ confidence in their treatment recommendation. Patient anxiety and decisional conflict were significantly lower after recurrence score results were known.
Apart from Oncotype DX, other multigene tests being used clinically to predict recurrence risk and guide adjuvant chemotherapy decisions include MammaPrint and PAM50. Prospective clinical trials are ongoing to further validate these assays. The TAILORx trial uses the Oncotype DX recurrence score to assign estrogen receptor (ER)-positive, node-negative patients to chemotherapy plus hormonal therapy vs hormonal therapy alone. The RxPONDER (SWOG S1007) trial uses Oncotype DX in a similar approach but for patients with one to three node-positive nodes. The MINDACT trial uses MammaPrint and Adjuvant! Online for treatment arm assignments. MINDACT has very broad eligibility criteria and two secondary randomizations for selecting chemotherapy and hormonal therapy regimens. Genome sequencing may potentially identify the molecular abnormalities that underlie the poor risk identified by multigene tests and provide potential new targets for therapy.
Chemotherapy. Multiagent therapy with CMF (cyclophosphamide, methotrexate, and fluorouracil [5-FU]), CMFP (cyclophosphamide, methotrexate, 5-FU, and prednisone), AC (Adriamycin [doxorubicin] and cyclophosphamide), and MF (sequential methotrexate and 5-FU) has been used in patients with node-negative disease (see Table 2 in the “Stages 0 and I Breast Cancer” chapter).
For node-positive disease, systemic chemotherapy has changed over the past few decades. Anthracycline-containing regimens have been shown to be of greater benefit than non–anthracycline-containing regimens (eg, CMF). Epirubicin (Ellence) was approved by the US Food and Drug Administration (FDA) for use in combination chemotherapy, in CEF (cyclophosphamide, epirubicin, and 5-FU) for adjuvant treatment of patients with node-positive breast cancer following resection of the primary tumor.
In a pivotal trial conducted by the NCIC, premenopausal women with node-positive breast cancer were randomly allocated to receive either CEF or CMF, administered monthly for 6 months. With a median follow-up of 59 months, the 5-year relapse-free survival rates were 53% and 63% ( P = .009), and 5-year survival rates were 70% and 77% ( P = .03) for CMF and CEF, respectively.
Several trials have also shown the benefit of incorporating taxanes (paclitaxel and docetaxel [Taxotere]) in the adjuvant treatment of node-positive breast cancer, and these drugs are now routinely used in this setting. Taxanes can either be given in combination with an anthracycline or sequentially, either before or after an anthracycline. They can also be given in combination with other drugs such as cyclophosphamide.
The E1199 trial compared paclitaxel with docetaxel and therapeutic schedules (ie, every 3 weeks vs weekly) in the adjuvant therapy of operable breast cancer. In all, 4,950 eligible patients with lymph node–positive or high-risk (tumor > 2 cm), node-negative breast cancer received 4 cycles of AC and then were randomized to receive IV paclitaxel or docetaxel given at 3-week intervals for 4 cycles or at 1-week intervals for 12 cycles. With a median follow-up of 12.1 years, adjuvant weekly paclitaxel (HR = 0.84; 95% CI, 0.73–0.96) and every-3-week docetaxel (HR = 0.79; 95% CI, 0.68–0.90) were associated with significantly improved disease-free survival compared to every-3-week paclitaxel when given sequentially following AC. Exploratory analysis in triple-negative breast cancer (n = 1,025) showed that the most effective taxane regimen was weekly paclitaxel, with an improvement in the 10-year disease-free survival rate from 59% to 69% and improvement in the overall survival rate from 66% to 75%. Among hormone-positive, HER2-negative/unknown disease (n = 2,785), the trend for improved outcomes initially seen at 5 years were not consistently observed. Furthermore, strong associations with inferior outcomes were observed for obese women and black race, independent of obesity.
The Breast Cancer International Research Group (BCIRG) compared TAC (Taxotere [docetaxel], AC) with the FAC regimen (5-FU, AC) in 1,480 women with node-positive breast cancer (BCIRG 001/TAX 316). At a median follow-up of 55 months, the estimated 5-year disease-free survival was 75% for patients treated with TAC vs 68% for those treated with FAC. This represents a statistically significant reduction in the risk of relapse [ P = .001]). Furthermore, treatment with TAC resulted in a statistically significant reduction in the risk of death (30%; P = .008). The improvement in both disease-free and overall survival was confirmed in the 10-year follow-up analysis of the BCIRG 001 trial (disease-free survival: HR = 0.80; 95% CI, 0.68–0.93; P = .0043 and overall survival: HR = 0.74; 95% CI, 0.61–0.90; P = .002). Although there was more febrile neutropenia with TAC, it was ameliorated with growth factor support. The rate of grade 3/4 congestive heart failure was greater in the TAC vs FAC group (3.5% vs 2.3%), as was the incidence of acute myeloid leukemia (four patients treated with TAC and two patients treated with FAC).
In the Cancer and Leukemia Group B (CALGB) trial 9344, a total of 3,121 women with operable, node-positive breast cancer were randomized to receive three different doses of doxorubicin with a standard dose of cyclophosphamide, followed by either no further therapy or 4 cycles of paclitaxel (at 175 mg/m 2 ). This study did not show any substantial benefit from dose escalation of doxorubicin. However, the addition of 4 cycles of paclitaxel improved disease-free and overall survival. At 5 years, the disease-free survival rates were 65% for the AC-treated cohort and 70% for the AC-plus-paclitaxel treatment group, and overall survival rates were 77% and 80%, respectively. An unplanned subset analysis showed that the majority of the benefit was seen in patients with estrogen receptor-negative tumors. Tamoxifen was given to 94% of patients with hormone receptor-positive tumors. Toxicity was modest with the addition of 4 cycles of paclitaxel.
Jones et al previously reported that 4 cycles of docetaxel and cyclophosphamide (TC) improved overall survival when compared with 4 cycles of AC in early breast cancer. Updated results of this study as well as the impact of age, hormone receptor status, and HER2 status on outcome and toxicity were published. Of note, 16% of patients in this trial were > 65 years. The median age in women under the age of 65 was 50 years (range, 27–64 years) and for women over age 65 was 69 years (range, 65–77 years). Baseline characteristics in the two age subgroups generally were well matched, except that older women tended to have more lymph node involvement. At a median of 7 years follow-up, the difference in disease-free survival between the TC and AC groups was significant (81% vs 75%, respectively; P = .033; HR = 0.74; 95% CI, 0.56–0.98), as was the difference in overall survival (87% vs 82%; P = .032; HR = 0.69; 95% CI, 0.50–0.97). The TC regimen was superior in both older and younger patients. Older women experienced more febrile neutropenia with TC and more anemia with AC. However, studies comparing TC with an anthracycline-and-taxane containing regimen (ie, TAC) are ongoing.


Endocrine therapy
The EBCTCG overview analyses demonstrated a significant advantage with the addition of tamoxifen (20 mg/d oral) for 5 years to the adjuvant therapy regimen of women with ER-positive breast cancer, regardless of age. Treatment with tamoxifen reduced the risk of death by 14% in women younger than age 50 and by 27% in those 50 years of age and older. Long-term follow-up from the NSABP conclusively demonstrates that there is no benefit to continuing tamoxifen therapy beyond 5 years. However, results from much larger studies have recently reported that a longer duration of tamoxifen may be more beneficial than 5 years of therapy.
From 1996 to 2005, the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) trial randomized 11,500 women (59% ER-positive, 41% untested) who had completed about 5 years of adjuvant tamoxifen to 5 more years of tamoxifen vs stopping. Less than 1% of patients had switched to any other adjuvant hormonal therapy in the trial treatment period. At a mean follow-up of 4.2 years, the annual recurrence rate in each treatment group was approximately constant during and after the 5-year trial treatment period. Approximately 1,500 recurrences have been reported. A total of about 1,300 occurred during years 5 to 9, but only about 200 occurred during years 10 to 14. Overall, the recurrence rate was significantly lower among patients allocated to continue tamoxifen. There was no significant heterogeneity in the recurrence rate reduction with respect to ER status, time period, age, or nodal status at diagnosis. Breast cancer mortality and overall mortality rates were lower among those allocated to continue tamoxifen, but were not statistically significant. Further follow-up is needed to reliably assess the longer-term effects on recurrence and the net effects on mortality.
Premenopausal women. Approximately 60% of premenopausal women with primary breast cancer have ER-positive tumors. For this group of patients, the benefit of adjuvant endocrine therapy, either tamoxifen or ovarian ablation, was established in the EBCTCG overview. For premenopausal women, however, the long-term morbidity associated with permanent ovarian suppression may be significant. Ovarian suppression with luteinizing hormone-releasing hormone (LHRH) analogs offers an alternative to permanent ovarian ablation, which is potentially reversible on cessation of therapy.
The ZEBRA (Zoladex Early Breast Cancer Research Association) trial is a randomized study that directly compares goserelin (Zoladex) monotherapy with CMF in premenopausal women 50 years of age and younger with node-positive, stage II breast cancer. The study included 1,614 patients: 797 randomized to receive goserelin and 817 to receive CMF. ER status was known for 92.5% of patients and 80% had ER-positive tumors.
At a median follow-up of 6 years, the ER-positive patients treated with goserelin fared comparably to those who received CMF in terms of disease-free survival (HR = 1.01; P = .94) and overall survival (HR = 0.99; P = .92). Not surprisingly, CMF was superior to goserelin in patients with ER-negative tumors. The onset of amenorrhea occurred on average 6 months sooner with goserelin than with CMF. More than 95% of patients on goserelin were amenorrheic vs 59% of patients receiving CMF. Reversibility of amenorrhea was greater for goserelin. One year after cessation of goserelin treatment, 23% remained amenorrheic vs 77% of CMF recipients.
Several studies have compared adjuvant chemotherapy with combined endocrine therapies, consisting of tamoxifen for 5 years and an LHRH agonist for 2 to 3 years, in premenopausal women. Overall, combination endocrine treatment yielded better results than did chemotherapy alone. Whether a strategy of combined endocrine therapy is better than tamoxifen alone, either with or without chemotherapy, in premenopausal patients with hormone receptor–positive tumors is the subject of several ongoing clinical trials.
Two randomized, phase III studies, SOFT (Suppression of Ovarian Function Trial) and TEXT (Tamoxifen and Exemestane Trial) investigated the role of ovarian suppression plus tamoxifen vs anaromatase inhibitor, exemestane, in the adjuvant therapy of premenopausal women with hormone receptor–positive breast cancer. Primary analysis of the combined data from both trials (n = 4,690) were reported by Pagani et al. With a median follow-up of 68 months, disease-free survival at 5 years was 91.1 in the exemestane-OFS group compared to 87.3% in the tamoxifen-OFS group (HR = 0.72; 95% CI, 0.60–0.85; P < .001). Overall survival was not statistically different between the two groups (HR = 1.14; 95% CI, 0.86–1.51; P = .37); however, conclusions about overall survival are premature at this early point in follow-up of hormone receptor–positive disease. The side effect profile of exemestane-OFS mirrors that seen with use of aromatase inhibitors in postmenopausal women.
Sidebar : Additional results from SOFT, specifically the comparison between tamoxifen monotherapy vs tamoxifen-OFS was recently presented at the SABCS annual meeting. For this analysis, 2,033 women were included, 53% of whom received chemotherapy in addition to the assigned hormonal therapy. The median age was 43 years, 35% had node-positive disease, 32% had tumors > 2 cm, 22% had grade 3 tumors, and 12% had HER2-positive disease. Results showed that the overall study population did not benefit from the addition of OFS to tamoxifen. The 5-year disease-free survival was 84.7% for tamoxifen alone vs 86.6% for tamoxifen-OFS (HR = 0.83; 95% CI, 0.66–1.04; P = .10). There was a 19% relative reduction in breast cancer recurrence between the tamoxifen-OFS group vs tamoxifen alone ( P = .09). Women who were < 35 years of age (11.5% of the study population) had the most striking benefit from OFS. It is hoped that ongoing translational studies would help to further identify which groups of patients would derive the most benefit from OFS (Francis P, presenting for SOFT investigators. 37th SABCS, December 9–13, 2014. Abstract S3-08) .
Whether combined endocrine therapies alone may be sufficient to achieve excellent outcomes without chemotherapy was investigated in the PERCHE (Premenopausal Endocrine Responsive Chemotherapy) trial. Chemotherapy use was determined by randomization; unfortunately, the trial closed due to inadequate accrual. In the TEXT clinical trial, in which chemotherapy use was chosen by the physician, lymph node status was the predominant determinant of chemotherapy use (88% of treated node-positive vs 46% of node-negative patients). Geography, patient age, and tumor size and grade were also determinants, but degree of receptor positivity and HER2 status were not. Currently, almost all premenopausal women with lymph node–positive, HR-positive breast cancer receive chemotherapy.
In premenopausal women with early breast cancer, addition of zoledronic acid (Zometa) to adjuvant endocrine therapy significantly improved clinical outcomes beyond endocrine therapy alone. In the Austrian Breast and Colorectal Cancer Study Group (ABCSG)-12 trial, a phase III randomized study of 1,801 premenopausal women with stage I–II disease undergoing ovarian suppression with goserelin, therapy with tamoxifen or anastrozole (Arimidex) plus zoledronic acid reduced the risk of disease-free survival events by 36% and relapse-free survival by 35% vs endocrine therapy alone ( P = .011 and P = .015, respectively). At a median follow-up of 62 months, Gnant et al reported that zoledronic acid reduced the risk of disease-free survival events (relapse) overall (HR = 0.68; 95% CI, 0.51–0.91; P = .009), although the difference was not significant in the tamoxifen (HR = 0.67; 95% CI, 0.44–1.03; P = .067) and anastrozole (HR = 0.68; 95% CI, 0.45–1.02; P = .061) arms assessed separately. Zoledronic acid did not significantly affect risk of death (HR = 0.67; 95% CI, 0.41–1.07; P = .09). There was no difference in disease-free survival between patients on tamoxifen alone vs anastrozole alone (HR = 1.08; 95% CI, 0.81–1.44; P = .591), but overall survival was worse with anastrozole than with tamoxifen (46 vs 27 deaths; HR = 1.75; 95% CI, 1.08–2.83; P = .02). Treatments were generally well tolerated, with no reports of renal failure or osteonecrosis of the jaw. Bone pain was reported in 601 patients (33%; 349 patients on zoledronic acid vs 252 not on the drug), fatigue in 361 (20%; 192 vs 169), headache in 280 (16%; 147 vs 133), and arthralgia in 266 (15%; 145 vs 121). The authors concluded there were persistent benefits with zoledronic acid and support its addition to adjuvant endocrine therapy in premenopausal patients with early-stage breast cancer.
Postmenopausal women. For many years, tamoxifen has been the gold standard adjuvant endocrine therapy for postmenopausal women with hormone receptor–positive tumors. However, after third-generation aromatase inhibitors demonstrated superior activity in metastatic breast cancer, large randomized clinical trials were initiated in patients with early-stage breast cancer to evaluate these drugs compared with tamoxifen, in combination with tamoxifen, and sequentially with tamoxifen.
The AZURE (Adjuvant Zoledronic Acid to Reduce Recurrence) trial is one of the largest phase III studies of adjuvant bisphosphonates designed to determine whether treatment with zoledronic acid (ZA) added to standard adjuvant therapy improves disease-free survival in a broader range of patients with stage II/III breast cancer. A total of 3,360 patients were randomized to receive chemotherapy and/or endocrine therapy (ET) with or without 4 mg ZA intravenously every 3 to 4 weeks for six doses, then three doses monthly × 8 and six doses monthly × 5 to complete 5 years of treatment. With a median follow-up of 59 months, there have been 752 disease-free survival events (ZA = 377 vs control = 375; HR = 0.98; 95% CI, 0.85–1.13; P = .79).
The numbers of deaths-243 in the ZA group and 276 in the control group-were also similar, resulting in overall survival rates of 85.4% in the ZA group and 83.1% in the control group (adjusted HR = 0.85; 95% CI, 0.72–1.01; P = .07). Subgroup analysis of premenopausal, ER-positive patients (n = 1,185), approximating the ABCSG 12 population, also gave no indication of benefit from ZA. Serious adverse events were similar in both treatment arms. There were 17 confirmed cases of osteonecrosis of the jaw (cumulative incidence, 1.1%; 95% CI, 0.6–1.7; P < .001) and 9 suspected cases; there were no cases in the control group. Rates of other adverse effects were similar in the two study groups.
ATAC (Arimidex, Tamoxifen, Alone or in Combination) was the first large, randomized trial demonstrating the superiority of an aromatase inhibitor over tamoxifen in the adjuvant treatment of postmenopausal women with HR-positive breast cancer. After the initial ATAC analyses, the combination arm was closed because of low efficacy.
ATAC has shown that anastrozole (n = 3,125) is significantly more effective than tamoxifen (n = 3,116) in preventing recurrences and is better tolerated but associated with a higher risk of fractures on treatment. After treatment completion, fractures and serious adverse events continued to be collected in a blinded fashion.
At a median follow-up of 100 months, the ATAC trial showed significant improvement for anastrozole compared with tamoxifen for disease-free survival, time to recurrence, time to distant recurrence, and contralateral breast cancer. In the HR-positive population, the results showing this benefit with anastrozole were: disease-free survival (HR = 0.85; 95% CI, 0.76–0.94; P = .003), time to recurrence (HR = 0.76; 95% CI, 0.67–0.87; P = .0001); time to distant recurrence (HR = 0.84; 95% CI, 0.72–0.97; P = .022); and incidence of new contralateral breast cancer (HR = 0.6; 95% CI, 0.42–0.85; P = .004). Absolute differences for anastrozole vs tamoxifen increased over time, and hazard rates remained lower on anastrozole compared with tamoxifen after treatment completion. Breast cancer deaths were nonsignificantly fewer with anastrozole than with tamoxifen (351 vs 380 intent-to-treat; 246 vs 268 hormone receptor–positive), but there was no difference in overall survival (HR = 0.97, hormone receptor–positive). After treatment completion, fracture rates for anastrozole and tamoxifen were similar, and safety benefits were maintained. Myocardial infarction rates among patients were identical to those seen on or off treatment, and endometrial cancer rates remained lower for anastrozole than for tamoxifen off treatment. No new safety concerns were seen. These data confirm the long-term superior efficacy and safety of anastrozole over tamoxifen as initial adjuvant therapy for postmenopausal women with hormone-sensitive early breast cancer.
Exploratory analysis from the ATAC trial investigated the impact of body mass index (BMI) on recurrence and the relative benefit of anastrozole vs tamoxifen according to baseline BMI. Overall, women with a high BMI (> 35 kg/m 2 ) at baseline had more recurrences than women with a low BMI (< 23 kg/m 2 ; adjusted HR = 1.39; 95% CI, 1.06–1.82; P [heterogeneity] = .03) and significantly more distant recurrences (adjusted HR = 1.46; 95% CI, 1.07–1.61; P [heterogeneity] = .01). The relative benefit of anastrozole vs tamoxifen was nonsignificantly better in thin women vs overweight women. Recurrence rates were lower for anastrozole than tamoxifen for all BMI quintiles. These results confirm the poorer prognosis of obese women with early-stage breast cancer and suggest that the relative efficacy of anastrozole compared with tamoxifen is greater in thin postmenopausal women. Requiring independent confirmation is the concept that higher doses or more complete inhibitors might be more effective in overweight women.
The use of an aromatase inhibitor as upfront adjuvant endocrine therapy for postmenopausal women with HR-positive breast cancer was confirmed in the Breast International Group (BIG) 1-98 trial. This study compared letrozole (Femara) with tamoxifen for 5 years as adjuvant endocrine therapy for this patient population. At a median follow-up time of 51 months for the monotherapy (non-crossover) arms, 352 disease-free survival events among 2,463 women receiving letrozole and 418 events among 2,459 women receiving tamoxifen were observed. This reflected an 18% reduction in the risk of an event (HR = 0.82; 95% CI, 0.71–0.95; P = .007). No predefined subsets showed differential benefit. Adverse events were similar to those noted in previous reports, with patients on tamoxifen experiencing more thromboembolic events, endometrial pathology, hot flashes, night sweats, and vaginal bleeding, and those on letrozole experiencing more bone fractures, arthralgia, low-grade hypercholesterolemia, and cardiovascular events other than ischemia and cardiac failure. The present updated analysis yielded results that were similar to those from the previous primary analysis but more directly comparable with results from other trials of continuous therapy using a single endocrine agent. The BIG 1-98 trial was later modified to include a crossover for both agents.
Goss et al reported on a subset of women in the MA17 trial who were premenopausal at initial diagnosis and in whom subsequent menopause, prior to randomization, may have influenced their outcome on extended adjuvant letrozole. Women randomized to MA17 were divided into two groups: (1) premenopausal: women < 50 years of age who underwent bilateral oophorectomy when tamoxifen treatment was started or women < 50 years of age at the start of tamoxifen treatment who became amenorrheic during adjuvant chemotherapy or tamoxifen treatment; and (2) postmenopausal. Disease-free survival from time of randomization for women in these two groups was compared; 889 women were identified as premenopausal and 4,277 were identified as postmenopausal. The interaction between treatment and menopausal status was statistically significant for disease-free survival ( P = .02), indicating that women diagnosed with premenopausal breast cancer had significantly greater benefit (HR = 0.25; 95% CI, 0.12–0.51) with letrozole treatment in terms of disease-free survival than those with postmenopausal status (HR = 0.69; 95% CI, 0.52–0.91). Letrozole was well tolerated in premenopausal women. These data indicate that women who are premenopausal at diagnosis but become postmenopausal any time before or during adjuvant tamoxifen should be considered for extended adjuvant therapy with letrozole.
At a median follow-up of 71 months after randomization in the BIG 1-98 study, the letrozole monotherapy arm was compared with the sequential-therapy arms. In terms of disease-free survival, there was no difference between the letrozole monotherapy, tamoxifen sequenced to letrozole, or letrozole-followed-by-tamoxifen arms. The letrozole-followed-by-tamoxifen arm had an HR of 0.96, with a 99% CI of 0.76–1.21, and the tamoxifen-followed-by-letrozole arm had an HR of 1.05, with a 99% CI of 0.84–1.32.
Other randomized trials have investigated the use of an aromatase inhibitor after tamoxifen. Two sequential strategies after tamoxifen were studied: (1) a switch to an aromatase inhibitor after 2 or 3 years of tamoxifen to complete a 5-year course of endocrine therapy, or (2) a switch to an aromatase inhibitor after 5 years of tamoxifen to complete 10 years of endocrine therapy, also called extended adjuvant therapy. With either strategy, use of an aromatase inhibitor after tamoxifen provided significant reduction in events (recurrence, contralateral breast cancer, or death).
In the IES (Intergroup Exemestane Study), 4,742 patients who had received 2 to 3 years of tamoxifen were randomized to receive either additional tamoxifen or a switch to exemestane to complete a 5-year course of endocrine therapy. After a median follow-up of 55.7 months, 809 events contributing to the analysis of disease-free survival had been reported (354 events in patients treated with exemestane, 455 in those who received tamoxifen); an unadjusted HR of 0.76 (95% CI, 0.66–0.88; P = .0001) was in favor of exemestane, with an absolute benefit of 3.3% (95% CI, 1.6–4.9) by the end of treatment (ie, 2.5 years after randomization). A total of 222 deaths occurred in the exemestane group compared with 261 deaths in the tamoxifen group, with an unadjusted HR of 0.85 (95% CI, 0.71–1.02; P = .08) in the intent-to-treat group. When 122 patients with ER-negative disease were excluded, the HR was 0.83 (0.69–1; P = .05). Results suggest that early improvements in disease-free survival noted in patients who switch to exemestane after 2 to 3 years on tamoxifen persist after treatment and translate into a modest improvement in overall survival.
Severe toxic events in patients on exemestane were rare, and toxicity profiles were generally similar to those previously reported for aromatase inhibitors. Patients who received exemestane reported fewer venous thromboembolic events than did those on tamoxifen. No other statistically significant differences in reported cardiovascular events (excluding venous thromboembolic events) were noted either on treatment or including the post-treatment period. Myocardial infarctions were rare and occurred in 31 (1.3%) exemestane-treated patients as compared with 19 (0.8%) tamoxifen-treated patients ( P = .08). Any effect of treatment on the risk of myocardial infarction seemed largely restricted to patients with a history of hypertension. Musculoskeletal pain, carpal tunnel syndrome, joint stiffness, paresthesia, and arthralgia were reported more frequently in patients who switched to exemestane than in those who remained on tamoxifen. These effects emerged during the on-treatment period. In total, fractures occurred in 277 patients, but hip, spine, and wrist fractures were few. Including on-treatment and post-treatment follow-up, other types of fractures were more common in patients who switched to exemestane than in those on tamoxifen. Fewer clinically serious gynecologic events were reported in patients who switched to exemestane than in those on tamoxifen in the on-treatment period and throughout follow-up. The number of endometrial cancers did not differ significantly between the groups.
Three other randomized trials showed a benefit to switching to anastrozole after 2 to 3 years of tamoxifen treatment vs continued tamoxifen for a total of 5 years. The ITA (Italian Tamoxifen Arimidex) trial, with 448 patients enrolled and a median follow-up of 36 months, showed significant benefits in event-free survival (HR = 0.35; 95% CI, 0.20–0.63; P = .0002) and recurrence-free survival (HR = 0.35; 95% CI, 0.18–0.68; P = .001) in the women switched to anastrozole. There were 19 total events in the tamoxifen group (n = 225) and 10 in the anastrozole group (n = 223). The 3-year difference in recurrence-free survival was 5.8% (95% CI = 5.2–6.4). Significantly longer locoregional recurrence-free survival (HR = 0.15; 95% CI, 0.03–0.65; P = .003) was noted for the anastrozole group. The difference in distant recurrence-free survival approached statistical significance (HR = 0.49; 95% CI, 0.22–1.05; P = .06).
A combined analysis of the ABCSG Trial 8 and the ARNO (Arimidex-Nolvadex) 95 Trial, with 3,224 patients and a median follow-up of 28 months, investigated a similar strategy. It showed that sequential endocrine therapy with tamoxifen for 2 years followed by anastrozole for 3 years was superior to 5 years of tamoxifen in terms of event-free survival (HR = 0.6; 95% CI, 0.44–0.81; P = .0009) and distant recurrence–free survival (HR = 0.61; 95% CI, 0.42–0.87; P = .0067). No statistically significant difference in overall survival has emerged at this point ( P = .16). Updated results from the ARNO 95 trial indicated that switching to anastrozole resulted in a significant reduction in the risk of disease recurrence (HR = 0.66; 95% CI, 0.44–1; P = .049) and improved overall survival.
In the MA17 trial, 5,187 postmenopausal women who had taken tamoxifen for 5 years were randomly assigned to receive either letrozole or placebo for an additional 5 years. At a median follow-up of 30 months, an updated analysis of MA17 was performed. It confirmed the results of the first interim analysis. There continued to be an improvement seen with letrozole in disease-free survival (HR = 0.58; 95% CI, 0.45–0.76; 2 P < .001) and distant disease–free survival (HR = 0.60; 95% CI, 0.43–0.84; P = .002).
Bone effects. The third-generation aromatase inhibitors have been shown to reduce bone mineral density (BMD) when compared with tamoxifen in the advanced adjuvant and neoadjuvant settings in women with early breast cancer. Five-year results from the ATAC trial showed that patients treated with anastrozole had an annual decline in lumbar BMD of 2% during the first 2 years of treatment and of 1% in years 3 to 5.
The bone subprotocol of IBIS-II (International Breast Cancer Intervention Study-II) assessed changes in the BMD in postmenopausal women aged 40 to 70 years with a high risk of breast cancer who received anastrozole or placebo for 5 years. To date, of the 1,540 women in the prevention study, 613 have taken part in the bone subprotocol of the study. Of the 250 women whose lumbar spine and femoral neck BMD has been assessed at baseline and 1 year by dual-energy x-ray absorptiometry (DEXA) scans, 162 with normal BMD received only monitoring without bisphosphonate treatment, 59 osteopenic women were further randomized to receive either risedronate (Actonel) or placebo, and 29 osteoporotic women received treatment with risedronate. Data from this trial confirm the BMD losses observed with third-generation aromatase inhibitors in breast cancer patients, but it is also reassuring that BMD loss can be controlled if women receive DEXA scans at baseline and bisphosphonate treatment as needed along with aromatase inhibitors.
ABCSG-12, described previously in this chapter, is a randomized, open-label, phase III, four-arm trial comparing tamoxifen (20 mg/d orally) and goserelin (3.6 mg every 28 days SC) with or without zoledronic acid (4 mg IV every 6 months) vs anastrozole (1 mg/d PO) and goserelin with or without zoledronic acid for 3 years in premenopausal women with endocrine-responsive breast cancer. The median patient age at diagnosis was 44 years. In a BMD subprotocol, patients underwent serial BMD measurements at 0, 6, 12, 24, 36, and 60 months. Of 1,801 patients in the trial, 404 were prospectively included in a bone substudy. A total of 201 patients received adjuvant zoledronic acid together with their endocrine treatment, whereas 203 patients did not. After 3 years of treatment, patients who did not receive zoledronic acid showed a BMD loss of 11.3% as compared with baseline ( P < .001). Bone loss was more pronounced if anastrozole was used in combination with goserelin as compared with tamoxifen (−13.6% vs −9%). At 60 months of follow-up (ie, 2 years after the completion of treatment), patients without zoledronic acid still showed impaired BMD as compared with baseline (−6.8%; P = .0005). In contrast, patients who received zoledronic acid showed unchanged BMD at 36 months (+ .3%; P = .85) and increased BMD at 60 months (+3.9%; P = .02).
Z-FAST (Zometa-Femara Adjuvant Synergy Trial) evaluated the efficacy and safety of zoledronic acid in preventing aromatase inhibitor–associated bone loss in postmenopausal women with early breast cancer who were receiving adjuvant letrozole therapy. A total of 602 patients with hormone receptor–positive early breast cancer starting letrozole were randomized to upfront zoledronic acid vs delayed zoledronic acid. The delayed group received zoledronic acid when either the post-baseline T-score decreased to below −2 or a clinical fracture occurred. All patients were treated with calcium and vitamin D.
The Z-FAST trial showed that the overall difference in the percentage change in BMD between the upfront and delayed zoledronic acid treatment groups, at both lumbar spine and total hip, progressively increased from baseline through 36 months. Therefore, administering zoledronic acid every 6 months for up to 36 months is effective in preventing bone loss associated with adjuvant aromatase inhibitor therapy in postmenopausal women with early breast cancer. At 36 months, the upfront zoledronic acid group (n = 189) showed a mean increase of 3.72% in lumbar spine BMD, whereas the delayed group (n = 188) showed a mean decrease of 2.95%, resulting in an absolute difference of 6.7% ( P < .001). The upfront group (n = 189) showed a mean increase of 1.66% in total hip BMD, whereas the delayed group (n = 187) showed a mean decrease of 3.51%, resulting in an absolute difference of 5.2% ( P < .001). The study was not designed to detect a significant difference in the fracture rate between treatment arms. Zoledronic acid was safe and well tolerated; no serious renal adverse events and no confirmed cases of osteonecrosis of the jaw were reported.
Recommendations. Guidelines from ASCO and the National Comprehensive Cancer Network (NCCN) highlight the appropriate use of aromatase inhibitors in postmenopausal women with hormone receptor-positive breast cancer. Aromatase inhibitors have a significant role in reduction of recurrence in early-stage breast cancer and should be included as part of the adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive disease. Using an aromatase inhibitor as upfront therapy or switching at some point after 2 to 3 years of tamoxifen is an acceptable strategy. Since the risk of breast cancer recurrence after completion of adjuvant endocrine therapy remains substantial, extended therapy with an aromatase inhibitor is another viable strategy for patients who are completing 5 years of tamoxifen. The extended use of adjuvant tamoxifen for 10 years rather than 5 years is also supported by two recently reported ongoing studies, the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial and the Adjuvant Tamoxifen - To Offer More? (aTTom) trial. Based on the results of ATLAS and aTTom, it would be reasonable in women who remain premenopausal after 5 years of adjuvant tamoxifen to have a discussion regarding the potential benefits of extending treatment to 10 years. Any such discussion should weigh the potential degree of benefit against the potential treatment side effects. In women who enter menopause during tamoxifen therapy, switching to an aromatase inhibitor or the use of an aromatase inhibitor upon completion of tamoxifen is another treatment option.
Sidebar : In the ATLAS trial, continuing tamoxifen to 10 years rather than stopping at 5 years produced a further reduction in recurrence and mortality, particularly after year 10, for women with ER-positive disease. Treatment allocation seemed to have no effect on breast cancer outcome among 1,248 women with ER-negative disease, and an intermediate effect among 4,800 women with unknown ER status. The cumulative risk of recurrence during years 5–14 was 21.4% for women allocated to continue versus 25.1% for controls; breast cancer mortality during years 5–14 was 12.2% for women allocated to continue versus 15% for controls (absolute mortality reduction 2.8%). Mortality without recurrence from causes other than breast cancer was not significantly affected by longer tamoxifen use. The cumulative risk of endometrial cancer during years 5–14 was 3.1% (mortality 0.4%) for women allocated to continue versus 1.6% (mortality 0.2%) for controls (absolute mortality increase 0.2%) (Davies C et al: Lancet 381:805–816, 2013) .
In the aTTom study, which recruited 6,953 women, those randomized to continue tamoxifen had a significant reduction in breast cancer recurrence (RR 0.85; 95% CI, 0.76–0.95; P = .003), with an absolute difference of 4% at 15 years from randomization. There was a nonsignificant reduction in breast cancer mortality (RR 0.88; 95% CI, 0.77–1.01; P = .06), with an absolute difference of 3% at 15 years from randomization. There was no obvious effect on non–breast-cancer mortality (RR 0.95; 95% CI, 0.84–1.08; P = .4). There was a significant excess of endometrial cancer in those who continued on tamoxifen (2.9% versus 1.3%; RR 2.20; 95% CI, 1.31–2.34; P < .0001), as well as deaths from endometrial cancer (1.1% vs 0.6%; RR 1.83; 95% CI, 1.09–3.09; P = .02). (Gray RG et al: J Clin Oncol 31(suppl):Abstract 5, 2013) .
In an analysis of the combined ATLAS and aTTom data (17,477 patients in total) there was a statistically significant reduction in breast cancer mortality (RR 0.85; 95% CI 0.77–0.94; P = .001) and improvement in overall survival (RR 0.91; 95% CI, 0.84–0.94; P = .008) with 10 years of tamoxifen .
Treatment of HER2-positive tumors
HER2-expressing breast cancers have been shown to have a worse outcome than their HER2-negative counterpart. Studies have demonstrated significant benefit from the addition of trastuzumab (Herceptin) to chemotherapy for both early-stage and metastatic breast cancer. In women with surgically resected breast cancer that overexpresses HER2, trastuzumab combined with chemotherapy improves disease-free and overall survival. Trastuzumab treatment decreases the risk of death by one-third ( P = .015) in patients with HER2-positive breast cancer. Cardiac toxicity is a potential side effect of trastuzumab therapy and is more prevalent in patients previously treated with doxorubicin. Trastuzumab should not be administered concurrently with doxorubicin because of an increased risk of cardiac toxicity. New York Heart Association (NYHA) Class III or IV congestive heart failure or death from cardiac causes at 3 years was seen in 4.1% of patients treated with doxorubicin and trastuzumab in the B-31 trial and in 2.9% of patients in the N9831 trial.
Four major trials of trastuzumab in the adjuvant setting have been published. The NSABP B-31 and the North Central Cancer Treatment Group (NCCTG) N9831 trials were jointly analyzed to include a total of 3,351 HER2-positive patients, with a median follow-up of 2 years (2.4 years in trial B-31 and 1.5 years in trial N9831). Both trials included two similar treatment arms: adjuvant chemotherapy with AC followed by paclitaxel with or without weekly trastuzumab for 1 year. Although there were differences between the two studies, including a third treatment arm in N9831 (sequencing trastuzumab after paclitaxel) that was not included in the joint analysis, the common question addressed was the effect of adding trastuzumab to AC followed by paclitaxel.
There were 261 events in the control group and 133 events in the trastuzumab group. The HR for a first event in the trastuzumab group, as compared with the control group, was 0.48 (95% CI, 0.39–0.59; P < .001). The percentages of patients alive and disease-free at 3 years were 75.4% in the control group and 87.1% in the trastuzumab group (absolute difference, 11.8%; 95% CI, 8.1–15.4). At 4 years, the respective percentages were 67.1% and 85.3% (absolute difference: 18.2%; 95% CI, 12.7–23.7). Distant metastases were reported in 193 patients in the control group and 96 in the trastuzumab group. The HR for a first distant recurrence was 0.47 in the trastuzumab group as compared with the control group (95% CI, 0.37–0.61; ( P < .001). At 3 years, 90.4% of women in the trastuzumab group were free of distant recurrence, as compared with 81.5% of women in the control group (absolute difference, 8.8%; 95% CI, 5.5–12.1); the respective rates at 4 years were 89.7% and 73.7% (absolute difference, 15.9%; 95% CI, 11.1–20.8). Both disease-free and overall survival were highly statistically significant for the trastuzumab-treated cohort.
Furthermore, there is an overall survival benefit to the addition of trastuzumab to chemotherapy. There were 62 deaths in the trastuzumab group, as compared with 92 deaths in the control group (HR = 0.67; 95% CI, 0.48–0.93; P = .015). The absolute survival rate at 3 years was 94.3% in the trastuzumab group and 91.7% in the control group (absolute difference, 2.5%; 95% CI, 0.1–5); at 4 years, the respective rates were 86.6% and 91% (absolute difference, 4.8%; 95% CI, 0.6–9). The principal adverse event associated with trastuzumab therapy among patients with prior exposure to anthracyclines is cardiac dysfunction.
In NSABP trial B-31, for patients initiated on trastuzumab therapy, the cumulative incidence of NYHA class III or IV CHF or death from cardiac causes at 3 years was 0.8% in the control group (4 patients had congestive heart failure, and 1 died of cardiac causes) and 4.1% in the trastuzumab group (31 patients had congestive heart failure). Of the 31 women in the trastuzumab group who had congestive heart failure, 27 have been followed for at least 6 months after the onset of heart failure, and only 1 reported persistent symptoms of heart failure at the most recent follow-up visit.
During treatment with paclitaxel alone or with trastuzumab, there was little imbalance between treatment groups in the incidence of any toxicity except for a higher incidence of left ventricular dysfunction in the trastuzumab group. Since the dramatic results are changing the way breast cancer is treated and many clinicians have adopted use of trastuzumab for similar groups of patients, the same monitoring used in these trials can be adopted in clinical practice to minimize cardiac toxicity. Additional toxicities were rare cases of interstitial pneumonitis, some of which appeared to be related to trastuzumab therapy. In trial B-31, four patients in the trastuzumab group had interstitial pneumonitis, and one of these patients died. In the N9831 trial, five patients in the trastuzumab group had grade 3+ pneumonitis or pulmonary infiltrates, and one of these patients died.
From May 2000 to April 2005 in the NCCTG N9831 trial, a total of 2,448 eligible women were enrolled for the comparison between Arm A: AC (doxorubicin [Adriamycin] plus cyclophosphamide) →T (paclitaxel [Taxol]) (n = 1,087) vs Arm B: AC→T→H (trastuzumab [Herceptin]) (n = 1,097). With 6-year median follow-up and 390 events, 5-year disease-free survival rates of 71.8% and 80.1% were seen for women in Arm A vs Arm B, respectively. Disease-free survival was significantly increased with trastuzumab added sequentially to paclitaxel (log-rank P < .001; arm B/arm A HR = 0.69; 95% CI, 0.57–0.85). Comparison of arm B (n = 954) and arm C (AC→T + H →H) (n = 949), with 6-year median follow-up and 313 events, revealed 5-year disease-free survival rates of 80.1% and 84.4% for Arms B and C, respectively. There was an increase in disease-free survival with concurrent trastuzumab and paclitaxel relative to sequential administration (Arm C/Arm B HR, 0.77; 99.9% CI, 0.53–1.11), but the P value (.02) did not cross the prespecified O’Brien-Fleming boundary (.00116) for the interim analysis. After adjusting for age, tumor size, number of positive nodes, and ER status, risk of disease progression was still found to be significantly decreased with the addition of trastuzumab following AC and then paclitaxel ( P < .001; adjusted Arm B/Arm A HR, 0.67; 95% CI, 0.54–0.81). In summary, disease-free survival is significantly improved with the addition of 52 weeks of trastuzumab (sequentially or concurrently) to AC→T. There is a statistically significant 33% reduction in the risk of an event with the sequential addition of H following AC→T. There is a strong trend for a 25% reduction in the risk of an event with starting H concurrently with T rather than sequentially after T. Therefore, based on a positive risk/benefit ratio, the investigators recommend that trastuzumab be incorporated in a concurrent fashion with T chemotherapy.
The international HERA (HERceptin Adjuvant) trial had a different design. It assessed HER2-positive patients who received a variety of chemotherapeutic regimens and were randomized to observation vs 1 or 2 years of every-3-week trastuzumab. Results were reported for only the 1 year of trastuzumab arm vs the observation arm, which included 5,081 patients with 1-year medical follow-up. Similar to the previously mentioned joint analysis, there was a reduction in observed events (ie, recurrence of breast cancer in the contralateral breast, P < .001) in favor of trastuzumab. This represents an absolute benefit in terms of disease-free survival at 2 years of 8.4%. Overall survival in the two groups was not significantly different (29 deaths with trastuzumab vs 37 with observation). Severe cardiotoxicity developed in 0.5% of the women who were treated with trastuzumab.
The incidence of cardiac adverse events was investigated in the HERA trial in patients treated with 1 year of trastuzumab; 1,698 patients were randomly assigned to observation and 1,703 randomly assigned to trastuzumab treatment; 94.1% of patients had been treated with anthracyclines. The incidence of discontinuation of trastuzumab because of cardiac disorders was 5.1%. At a median follow-up of 3.6 years, the incidence of cardiac endpoints remained low, though it was higher in the trastuzumab group than in the observation group (severe congestive heart failure, 0.8% vs 0%; confirmed significant left ventricular ejection fraction decreases, 3.6% vs 0.6%). In the trastuzumab group, 59 of 73 patients with a cardiac endpoint reached acute recovery; of these 59 patients, 52 were considered by the cardiac advisory board to have a favorable outcome from the cardiac endpoint. The cumulative incidence of any type of cardiac endpoint increases during the scheduled treatment period, but it remains relatively constant thereafter.
The BCIRG 006 study evaluated the benefit of adjuvant trastuzumab in 3,222 patients with HER2-positive breast cancer. Unique to this study was a nonanthracycline-containing regimen, which was expected to minimize the cardiotoxicity seen with trastuzumab (H) following anthracycline-based chemotherapy. There were three treatment arms: (1) AC (doxorubicin [Adriamycin] plus cyclophosphamide) followed by T (docetaxel [Taxotere]); (2) AC followed by TH (docetaxel plus trastuzumab [Herceptin]); and (3) TCH (docetaxel, carboplatin, trastuzumab).
At the latest analysis of BCIRG 006, a total of 656 disease-free survival events were observed (257 in the group receiving AC→T, 185 in the group receiving AC→T plus trastuzumab, and 214 in the group receiving TCH. During a median follow-up of 65 months, 348 patients had died. A significant benefit with respect to disease-free and overall survival was seen in both groups treated with trastuzumab-containing regimens, as compared with the group that received AC→T (standard therapy), which had a 5-year disease-free survival rate of 75% and an overall survival rate of 87%. For patients receiving AC→T plus trastuzumab, the 5-year rate of disease-free survival was 84% (HR for the comparison with AC→T, 0.64; P < .001), and the overall survival rate was 92% (HR = 0.63; P < .001). For patients receiving TCH, the 5-year rate of disease-free survival was 81% (HR = 0.75; P = .04), and the rate of overall survival was 91% (HR = 0.77; P = .04). In contrast, no significant difference in the rate of disease-free or overall survival was seen between the two trastuzumab-containing regimens. Benefit for trastuzumab was seen in both node-negative and node-positive patients; however, the benefit of trastuzumab was seen in node-positive patients at highest risk for recurrence (ie, those with at least four positive nodes), for whom the 5-year rate of disease-free survival was 73% in the group receiving AC→TH and 72% in the group receiving TCH, as compared with 61% in the group receiving AC→T (HR = 0.66; P = .002 for both comparisons). The incidence of congestive heart failure in the two trastuzumab-containing regimens was higher in the group receiving AC-T plus trastuzumab (2%) than in the AC→T group (0.7%) or the TCH group (0.4%); the incidence with AC→TH as compared with TCH was increased by a factor of 5. The difference in rates of congestive heart failure between the two trastuzumab-containing regimens significantly favored TCH over AC→TH ( P < .001). Finally, acute leukemias developed in seven patients who were treated with anthracycline-based regimens and in one patient who was treated with TCH but subsequently received an anthracycline for the treatment of a B-cell lymphoma that occurred after her breast cancer.
There are unresolved questions about the adjuvant use of trastuzumab, including the optimal duration of treatment, and long-term safety in this setting. Studies are also ongoing to assess the benefit of additional and/or combined anti-HER2 therapy with trastuzumab in the adjuvant setting.
Sidebar : Pertuzumab in combination with trastuzumab and docetaxel for neoadjuvant use in HER2-positive early-stage breast cancer received accelerated approval by the US Food and Drug Administration (FDA) in October 2013. The primary study used to support this combination was a multicenter, randomized phase II trial, reported by Gianni et al, that evaluated four neoadjuvant regimens. Treatment-naive women with HER2-positive breast cancer were randomized prior to surgery (NeoSphere trial). A total of 417 eligible patients were randomly assigned (1:1:1:1) to receive four neoadjuvant cycles of: trastuzumab (8 mg/kg loading dose, followed by 6 mg/kg every 3 weeks) plus docetaxel (75 mg/m 2 , escalating, if tolerated, to 100 mg/m 2 every 3 weeks; group A) or pertuzumab (loading dose 840 mg, followed by 420 mg every 3 weeks) and trastuzumab plus docetaxel (group B) or pertuzumab and trastuzumab (group C) or pertuzumab plus docetaxel (group D). The primary endpoint, examined in the intention-to-treat population, was pathological complete response (pCR) in the breast. Results showed the highest pCR rate in the group treated with a combination of pertuzumab, trastuzumab, and docetaxel compared with the other three groups. Patients given pertuzumab and trastuzumab plus docetaxel (group B) had a significantly improved pathological complete response rate (49 of 107 patients; 45.8% [95% CI = 36.1–55.7]) compared with those given trastuzumab plus docetaxel (group A; 31 of 107; 29% [20.6–38.5]; P = .0141). Among women given pertuzumab plus docetaxel (group D), 23 of 96 (24% [15.8–33.7]) had a pathological complete response, as did 18 of 107 (16.8% [10.3–25.3]) given pertuzumab and trastuzumab (group C). The magnitude of improvement in the pCR rate was higher in patients with hormone receptor–negative cancers. A major adverse event with the addition of pertuzumab was the increased incidence of left ventricular cardiac dysfunction .
Sidebar : In a multicenter, open-label phase II study looking at the safety of pertuzumab in the neoadjuvant setting (TRYPHAENA study), patients with operable, locally advanced, or inflammatory breast cancer were randomized 1:1:1 to receive six neoadjuvant cycles q3wk (Arm A: 5-fluorouracil, epirubicin, cyclophosphamide [FEC] + H + P × 3 → docetaxel [T] + H + P × 3; Arm B: FEC × 3 → T + H + P × 3; Arm C: T + carboplatin + H [TCH] + P × 6). pCR was assessed at surgery and adjuvant therapy given to complete 1 year of H. A total of 225 patients were randomized. During neoadjuvant treatment, 2 patients (2.7%; Arm B) experienced symptomatic left ventricular systolic dysfunction (LVSD) and 11 patients (Arm A: 4 [5.6%]; Arm B: 4 [5.3%]; Arm C: 3 [3.9%]) had declines in left ventricular ejection fraction of ≥ 10% points from baseline to < 50%. Diarrhea was the most common adverse event. The combination of P with H and standard chemotherapy resulted in low rates of symptomatic LVSD (Schneeweiss NE et al: Ann Oncol 24:2278–2284, 2013) .
Toxic effects of medical therapy
Chemotherapy. The most frequent acute toxicities are nausea/vomiting, alopecia, and hematologic side effects such as leukopenia and thrombocytopenia, as well as allergic reactions, cystitis, stomatitis, and nail/skin changes. Neutropenia, with its risk of infection, is a potentially life-threatening complication that requires prompt medical attention and broad-spectrum antibiotics until hematologic recovery occurs.
Other toxicities may include transient or permanent amenorrhea, infertility, early menopause, neuropathy, and leukemia. Amenorrhea is drug- and dose-related and is often permanent in women older than age 40. Recent evidence demonstrates that chemotherapy-induced ovarian failure in the adjuvant chemotherapy setting is associated with a high risk of rapid bone demineralization in the first 6 to 12 months after treatment. Thus, premenopausal women undergoing adjuvant chemotherapy must be closely evaluated to prevent the development of early osteoporosis. Cardiac failure, although rare, is potentially life-threatening and may be irreversible.
Endocrine therapy. Toxicities with tamoxifen or aromatase inhibitors include hot flashes, menstrual irregularities, vaginal discharge (tamoxifen), vaginal dryness (aromatase inhibitors), and weight gain. Thrombophlebitis and endometrial hyperplasia are more common with tamoxifen. Arthralgias, osteoporosis, and fractures are more common with aromatase inhibitors, although the incidence of hip fractures is low.
Follow-Up of Long-Term Survivors
There is no consensus among oncologists as to the appropriate and optimal follow-up routine for long-term breast cancer survivors. Recommendations for follow-up testing vary. The vast majority of relapses, both locoregional and distant, occur within the first 3 years. Surveillance is most intensive in the initial 5 years; thereafter, the frequency of follow-up visits and testing is reduced ( Table 2 ).
Recommendations
History and physical examination.
Surveillance methods include a detailed history and physical examination at each office visit. They are performed every 4 to 6 months for 5 years after completion of initial therapy, then annually thereafter. Patients at higher risk of recurrence or complications of treatment may require surveillance at shorter intervals. Patients who have been treated by mastectomy can be seen in the office annually after they have been disease-free for 5 years. Patients who were treated with breast-conserving surgery and radiotherapy can be followed at 6-month intervals until they have been disease-free for 6 to 8 years, after which time they can be assessed annually.
TABLE 2: Follow-up recommendations for asymptomatic long-term breast cancer survivors as per NCCN guidelines
Approximately 71% of breast cancer recurrences are detected by the patients themselves, and they will report a change in their symptoms when questioned carefully. In patients who are asymptomatic, physical examination will detect a recurrence in another 15%. Therefore, a patient’s complaint on history or a new finding on physical examination will lead to the detection of 86% of all recurrences.
Mammography
Mammography should be performed annually in all patients who have been treated for breast cancer. For patients who have undergone breast-conserving surgery, the first follow-up mammogram should be performed approximately 6 months after completion of radiation therapy. The risk of developing contralateral breast cancer is approximately 0.5% to 1% per year. In addition, approximately one-third of IBTRs in patients who have been treated by conservation surgery and radiotherapy are detected by mammography alone. As the time interval between the initial therapy and follow-up mammography increases, so does the likelihood that local breast recurrence will develop elsewhere in the breast rather than at the site of the initial primary lesion.
Chest x-ray
Routine chest radiographs detect between 2.3% and 19.5% of recurrences in asymptomatic patients and may be indicated on an annual basis.
Liver function tests
Liver function tests detect recurrences in relatively few asymptomatic patients, and their routine use has been questioned. However, these tests are relatively inexpensive, and it may not be unreasonable to obtain them annually.
Tumor markers
There is no evidence that tumor markers, such as carcinoembryonic assay, CA-15-3, and CA-57-29, provide an advantage in survival or palliation of recurrent disease in asymptomatic patients. Therefore, use of tumor markers to follow long-term breast cancer survivors is not recommended.
Postoperative bone scans are also not recommended in asymptomatic patients. In the NSABP B-09 trial, in which bone scans were regularly performed, occult disease was identified in only 0.4% of patients.
Liver and brain imaging
Imaging studies of the liver and brain are not indicated in asymptomatic patients. Position emission tomography scans are not routinely recommended. Their utility is primarily as an adjunct study, often to establish the extent of metastatic disease.
Pelvic examinations
Women with intact uteri who are taking tamoxifen should have yearly pelvic examinations because of their risk of tamoxifen-associated endometrial carcinoma, especially among postmenopausal women. The vast majority of women with tamoxifen-associated uterine carcinoma have early vaginal spotting, and any vaginal spotting should prompt rapid evaluation. However, since neither endometrial biopsy nor ultrasonography has demonstrated utility as a screening test in any population of women, routine use of these tests in asymptomatic women is not recommended.
Bone density
Premenopausal women who become permanently amenorrheic from adjuvant chemotherapy and postmenopausal women who are treated with an aromatase inhibitor are at increased risk for bone fracture from osteopenia/osteoporosis. These patients should undergo monitoring of bone health every 1 to 2 years.
Suggested Reading
Boughey JC, Suman, VJ, Mittendorf EA, et al: Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 310:1455–1461, 2013.
Coates AS, Keshaviah A, Thurlimann B, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 25:486–492, 2007.
Coleman RE, Marshall H, Cameron D, et al, for the AZURE investigators: Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 365:1396–1405, 2011.
Coombes RC, Kilburn LS, Snowdon CF, et al: Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 369:559–570, 2007.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, et al: Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet 378:1707–1716, 2011.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, et al: Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444, 2012.
Gianni L, Pienkowski T, Im YH, et al: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32, 2012.
Giuliano AE, Hunt KK, Ballman KV, et al: Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 305:569–575, 2011.
Gnant M, Mlineritsch B, Schippinger W, et al: Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360:679–691, 2009.
Goldstein LJ, Gray R, Badve S, et al: Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26:4063–4061, 2008.
Goss PE, Ingle JN, Martino S, et al: Outcomes of women who were premenopausal at diagnosis of early stage breast cancer in the NCIC CTG MA17 trial. Program and abstracts of the 32nd Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, Texas. Abstract 13.
Goss PE, Ingle JN, Pater JL, et al: Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol 26:1948–1955, 2008.
Gnant M, Mlineritsch B, Stoeger H, et al: Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 12:631–641, 2011.
Jones S, Holmes FA, O’Shaughnessy J, et al: Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27:1177–8311, 2009.
Kaufmann M, Jonat W, Hilfrich J, et al: Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 Study. J Clin Oncol 25:2664–2670, 2007.
Martin M, Pienkowski T, Mackey J, et al: Breast Cancer International Research Group 001 Investigators: Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313, 2005.
Martin M, Pienkowski T, Mackey J, et al: Ten-year follow-up analysis of the BCIRG 001 trial confirms superior disease-free and overall survival benefit of adjuvant TAC (docetaxel, doxorubicin, cyclophosphamide) over FAC (fluorouracil, doxorubicin, cyclophosphamide) in women with operable, node-positive breast cancer. Cancer Res 70(24 Suppl): Abstract S4-3, 2010.
Pagani O, Regan MM, Walley BA, et al: Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371:107–118, 2014.
Perez EA, Suman, VJ, Davidson NE, et al: Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 29:4491–4497, 2011.
Peto R, Davies C, on behalf of the ATLAS Collaboration: ATLAS (Adjuvant Tamoxifen, Longer Against Shorter): International randomized trial of 10 versus 5 years of adjuvant tamoxifen among 11,500 women--preliminary results. Breast Cancer Res Treat 106(Suppl): Abstract 48, 2007.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al: Herceptin Adjuvant (HERA) Trial Study Team: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672, 2005.
Pierce LJ, Hutchins LF, Green SR, et al: Sequencing of tamoxifen and radiotherapy after breast-conserving surgery in early-stage breast cancer. J Clin Oncol 23:24–29, 2005.
Procter M, Suter TM, de Azambuja E, et al: Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 28:3422–3428, 2010.
Romond EH, Perez EA, Bryant J, et al: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684, 2005.
Shulman LN, Cirrincione C, Berry DA, et al: Four vs 6 cycles of doxorubicin and cyclophosphamide (AC) or paclitaxel (T) as adjuvant therapy for breast cancer in women with 0-3 positive axillary nodes: CALGB 40101 a 22 factorial phase III trial: First results comparing 4 vs 6 cycles of therapy. Cancer Res 70(24 Suppl): Abstract S6-3, 2010.
Slamon D, Eiermann W, Robert N, et al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283, 2011.
Sparano JA, Wang M, Martino S, et al: Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671, 2008.
Sparano JA, Zhao F, Martino S, et al: Ten year update of E1199: Phase III study of doxorubicin-cyclophosphamide followed by paclitaxel or docetaxel given every 3 weeks or weekly in patients with axillary node-positive of high-risk node-negative breast cancer. Program and abstracts of the 37th San Antonio Breast Cancer Symposium; December 9–13, 2014; San Antonio, Texas. Abstract S3-03.
Swain SM, Tang G, Geyer CE, et al: Definitive analysis of a randomized adjuvant trial comparing dose-dense (DD) AC→paclitaxel (P) plus gemcitabine (G) with (DD) AC→P and with docetaxel, doxorubicin, and cyclophosphamide (TAC) in women with operable, node-positive breast cancer. J Clin Oncol 30(suppl): Abstract LBA1000, 2012.
Swain SM, Tang G, Geyer CE Jr, et al: Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol 31:3197–3204, 2013.
Whelan TJ, Olivotto I, Ackerman I, et al: NCIC-CTG Ma.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol 29(suppl): Abstract LBA1003, 2011.
Winer EP, Hudis C, Burstein HJ, et al: American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report. J Clin Oncol 23:619–629, 2005.
COVID-19 and Chemotherapy Association Holds in Pediatric Leukemia/Lymphoma
Pediatric patients with acute lymphoblastic leukemia/lymphoma were less likely to have severe COVID-19 infection.

Finding Ways to Break the Mold in GU Oncology
Leaders in genitourinary oncology spoke about key research advances as well as personal experiences in navigating the field.

FDA Gives Fast Track Designation to LYT-200 in Advanced Head and Neck Cancers
LYT-200 is currently being investigated for those with solid tumors and hematologic malignancies.

Achieving Health Equity in Lung Cancer Surgery
Rian M. Hasson Charles, MD, MPH, FACS, discusses advances in equitable lung cancer screening and her experiences as a woman in thoracic oncology.
Microwave Ablation Shows Noninferiority to Surgery in Thyroid Cancer
Microwave ablation may be a go-to treatment option instead of surgery for patients with multifocal T1N0M0 papillary thyroid cancer.
Real-World Data Support SLNB Omission in HR+ HER2– Breast Cancer
Oncologic outcomes from a real-world analysis appear to be comparable with those observed in the sentinel lymph node biopsy arm of the SOUND trial.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Close the Gap
- SurvivorNetTV
- Clinical Trials
Brave Baywatch Star, 52, Goes Bald For Cancer Treatment– Nicole Eggert Is Battling Stage 4 Breast Cancer SHARE
- Acute Myeloid Leukemia (AML)
- Bladder Cancer
- Breast Cancer
- Chronic Lymphocytic Leukemia (CLL)
- Chronic Myeloid Leukemia (CML)
- Colon Cancer
- Esophageal Cancer
- Heart Failure
- Hypertrophic Cardiomyopathy (HCM)
- Liver Cancer
- Lung Cancer
Multiple Myeloma
Discover local multiple myeloma resources:.
- Multiple Sclerosis (MS)
- Myelodysplastic Syndrome (MDS)
- Myeloproliferative Neoplasms
- Non-Hodgkin Lymphoma
Ovarian Cancer
Discover local ovarian cancer resources:.
- Prostate Cancer
- Rare Diseases Cold Agglutinin Disease (CAD) Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Von Hippel-Lindau Disease
- SN Guides: Critical information from SurvivorNet's experts
- Mental Health: Helping patients find the balance critical to treatment and recovery
- Clinical Trial Finder: Search for clinical trials that fit your profile
- Prevention & Screening
- Symptoms & Diagnosis
- Later Stage Treatment
- Living with Cancer
Multiple Myeloma: Main Page
Discover local multiple myeloma resources:, ovarian cancer: main page, discover local ovarian cancer resources:.
- Cold Agglutinin Disease (CAD)
- Mental Health
- Transthyretin Amyloid Cardiomyopathy (ATTR-CM)
- Von Hippel-Lindau Disease
- Newsletters and Guides
Brave Baywatch Star, 52, Goes Bald For Cancer Treatment– Nicole Eggert Is Battling Stage 4 Breast Cancer
- Save This Video
Finding Strength to Endure Breast Cancer Fight
- “Baywatch” star Nicole Eggert, 52, shares an empowering video on social media of herself cutting off her hair amid ongoing treatment for stage 2 breast cancer.
- Losing your hair or seeing it thinning is often a side effect of some cancer treatments. It usually begins about three to four weeks after chemotherapy and continues throughout treatment.
- Many people going through cancer treatment feel a sense of dread associated with losing their hair, making this stage of the journey very emotional. Such a drastic physical change may lead to anxiety and sleepless nights.
- Fortunately, your hair regrows shortly after finishing treatment, but the experience can bring a lot of emotions for women. New York-based psychiatrist Dr. Samantha Boardman suggests connecting with others in a similar situation to help cope.
- While no treatments guarantee your hair won’t fall out during or after chemotherapy, scalp-cooling caps and minoxidil (Rogaine) may help.
Hair loss is one of the most challenging stages of a cancer journey because hair is so emotionally connected to one’s identity. To share this often-private moment with her fans shows Eggert’s bravery and courage.
“Bad. Ass. Yes, you are. And stunningly gorgeous with or without hair!” podcaster Erika Eleniak commented on Instagram.
“Beautiful! You got this so many of us are on the other side and are doing great! You will there as well!” Instagram user Olivia Grochowski commented.
“Still a badass beauty,” Instagram user Demond Hawkins commented.
Resources on Hair Loss Options During Cancer Treatment
- Coping with Hair Loss During Ovarian Cancer Treatment
- If You’re Looking For Ways To Deal With Hair Loss During Cancer, You’re Not Alone
- How to Slow Hair Loss During Chemotherapy for Ovarian Cancer
How to Navigate One of the Most Emotional Steps During a Cancer Journey
Hair loss is challenging for women and men alike, but it can be incredibly difficult for cancer patients. Losing your hair or seeing it thinning is a common side effect of some cancer treatments.
Chemotherapy can cause hair loss. It usually begins about three to four weeks after starting chemotherapy and continues throughout treatment.
It happens because this treatment targets quickly dividing cells throughout the body. That includes cancer cells but also hair cells.
“For cancer patients, losing one’s hair can be unbelievably stressful. To start with, the dread of losing one’s hair can lead to some sleepless nights and feelings of anxiety,” Dr. Samantha Boardman , a New York-based psychiatrist and author, told SurvivorNet.
WATCH: Hair loss during chemo.
To better cope with this emotional stage of the journey, Dr. Boardman suggests reaching out to other survivors who have been through a similar situation.
Radiation is another treatment that can lead to hair loss if the hair is in the path of the tumor being treated. Radiation for a brain tumor, for example, may cause hair loss.
“If you do lose hair, it will regrow several weeks or months after treatment,” radiation oncologist Dr. James Taylor told SurvivorNet.
“Fortunately, for most patients, hair loss is not a concern when having radiation therapy,” Dr. Taylor continued.
Fortunately, hair loss during cancer treatment is not all bad news. Most people can expect regrowth four to six weeks after treatment. However, when your hair grows back, you may notice some changes in its color and texture.
If losing your hair is a concern for you before cancer treatment, know you have options like wigs, hats, wraps, and scarves, among other things.
Another option that can minimize hair loss is cryotherapy, “just a fancy way for saying cold therapy,” says Dr. Renata Urban, gynecologic oncologist at the University of Washington in Seattle.
Cryotherapy involves wearing cold caps or special cooling caps before, during, and after each chemotherapy treatment.
WATCH: What is a scalp-cooling device?
The caps, which are tightly fitting and strap-on helmet-style, are filled with gel coolant chilled to negative (-15) to (-40) degrees Fahrenheit.
The caps “cause vasoconstriction, or a narrowing of the blood vessels bringing blood to the scalp,” Dr. Urban explains. By constricting the blood flow to the scalp, the caps limit the amount of circulating chemotherapy that reaches the hair follicles, protecting them from some of the chemo’s damaging effects.
The cold also decreases the activity of the hair follicles, slowing down cell division and making them less affected by chemotherapy medicine.
“This has been shown to reduce hair loss by 50 percent,” Dr. Urban says. “I do try to let patients know it’s not a 100 percent prevention strategy, and it’s not been studied in all hair types, but it is at least an available strategy for patients to try.”
Keep in mind you will be experiencing some cold temperatures. Some women find the caps give them a headache. To help withstand the chilly temps, some women will dress warmly and bring blankets.
Remember to always talk with your doctor about potential treatments to mitigate the loss and the resources you have available to handle it.
Eggert’s Brave Cancer Journey
Eggert was diagnosed with stage 2 cribriform carcinoma breast cancer after discovering a lump in her breast while performing a self-breast exam . This type of exam is an easy way to keep watch for anything abnormal regarding your breasts. It involves feeling the breast for any swelling, bulging, or changes in the shape of the breast or nipple. Checking for signs of redness, rashes, or discharge is also part of this exam. If anything is found to be concerning, you should contact your doctor. It’s important to note that self-exams should be done with regular mammograms.
View this post on Instagram A post shared by Nicole Eggert (@_nicole_eggert)
Eggert said she began experiencing “terrible pain” and rapid weight gain that she first dismissed for signs of menopause. However, after she discovered a lump during a self-exam, a mammogram and multiple biopsies confirmed that she had breast cancer.
The rare type of breast cancer Eggert has is often slow-growing and low-grade, according to Breast Cancer Now.
“I can definitely feel it. It’s there. It needs to be taken out. So it’s just a matter of do I have to do treatment before the surgery or can they perform the surgery and then I do the treatment after,” Eggert told People.
Eggert is anticipating chemotherapy and radiation therapy for treatment.
The best course of treatment will likely be determined based on the initial tests the “Charles in Charge” star received upon her diagnosis.
Questions to Ask Your Doctor
If you’re going through cancer treatment and experiencing hair loss, here are some questions you may consider asking your doctor:
- Are there any treatments to help manage or minimize my hair loss?
- What are scalp-cooling devices, and how do they work?
- Do you recommend scalp-cooling devices?
- What other options are available to help me cope with hair loss?
- Can you recommend a wig maker?
- I’m struggling mentally with my hair loss; can you recommend a therapist to talk to?
- How can I find a local support group with people going through similar things?
Learn more about SurvivorNet's rigorous medical review process.
Kavontae Smalls is a writer and reporter for SurvivorNet. Read More
Your treatment can be guided by factors including hormones, genetics, and the specific characteristics of your specific cancer.
Patient Pathfinder:
Clinical Trial Finder
Are you looking for other treatment options? Right now there are ... breast cancer trials in the U.S. within 100 miles of
Enter your zip code and travel distance to explore trials in your area...
Directly affected by breast cancer? Stay informed. Stay hopeful.
Get critical information from SurvivorNet’s experts and hear stories of hope from survivors who’ve gotten through it.
You’re signed up…
The more we know about you the better we can help..
Get local perspectives. Connect with experts in your area.
Where are you located?
Hear from survivors and experts that match your profile. Which topics are you interested in?
Select topics:
You will soon recieve content from survivors and experts that matches your profile..
Explore: Prevention & Screening
Related Articles
Grappling With Thinning Hair, Former Victoria’s Secret Model Nicole Weider, 38, Embraces Wig Shopping Amid Hair Loss Journey During Cancer Treatment
Embracing Shaved Head, ‘Real Housewives of Miami’ Star Guerdy Abraira– Her Empowering Choice After Breast Cancer Treatment
Famous ‘Hairy TV Biker’ Rides Again— The 66-Year-Old Beating Cancer & Extreme Weight Loss
Star of Hit TV Dance Show, 33, ‘Feeling Much Better’ After Emergency Trip To Hospital– Overcoming Setbacks During Cancer Treatment
Get SurvivorNet in Your Inbox
Our newsletter – vital information, hope, and healing, delivered weekly.
Introducing, the Journey Bar
Use this bar to access information about the steps in your cancer journey.
Please sign in to save videos. Don't have an account? Register
Username or email address *
Remember me
Lost your password?
Not a member yet? Register now.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 12, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Softer tumors fuel more aggressive spread of triple-negative breast cancer, research shows
by Garvan Institute of Medical Research
Researchers have discovered how the mechanical properties of tumors can prime cancer cells to better survive their spread to other organs.
A metabolic 'survival switch' controlled by the stiffness of triple-negative breast tumors can significantly influence how successfully their cancerous cells spread to other organs, according to new findings from the Garvan Institute of Medical Research.
The study in cell and mouse models showed that softer tumor environments, typical of early-stage cancer, can prime triple-negative breast cancer cells to use an extra energy source for survival during metastasis. The research suggests that drugs targeting this altered cancer cell metabolism could boost treatments for metastatic triple-negative breast cancer.
"Our research suggests triple-negative breast cancer cells in soft tissue environments are 'primed' to better survive the spread to other organs and that they switch on an alternative form of metabolism to do so," says Associate Professor Cox, Head of the Matrix & Metastasis Lab at Garvan and senior author of the study published in Advanced Science .
"This suggests that triple-negative breast cancer cells spreading from softer tumors are more aggressive, and drugs that target cancer cell metabolism may benefit patients with metastatic triple-negative breast cancer treatment."
A metabolic survival advantage
Triple-negative breast cancers are highly aggressive and difficult to treat as they lack three receptors (for estrogen, progesterone and the HER2 protein) that can be targeted in other breast cancers. New treatment options are urgently needed for the 2,500 women diagnosed every year in Australia alone.
Using biomaterials that mimic the properties of tumors, the team investigated how triple-negative breast cancer cells respond to the physical stiffness of their environment. The researchers found the cancer cells were primed to be more resilient when grown in soft environments and, when injected into mouse models, up to 11.8 times more likely to metastasize to new sites compared to those from rigid tumor environments.
The team also discovered that soft environments altered the cancer cells' preference for 'fuel' in a way that enhanced their durability while traveling through the body. These primed cells metabolized glucose—the preferred energy source for cancer cells—but they also stockpiled lipids as internal fuel reserves and in turn ramped up lipid metabolism —a more resilient energy pathway for their journey from a primary tumor site.
"This switch to using both glucose and fats as an energy source equips cells to better survive the mechanical stresses of traveling through the bloodstream and seeding new tumor sites throughout the body," says first author Dr. Elysse Filipe, who completed the study as a postdoctoral researcher at Garvan.
"By blocking lipid metabolism in triple-negative breast cancer cells, we were able to 'starve' their high energy demand and reduce metastasis in a cell model."
A new approach for triple-negative breast cancer
"Our findings highlight that the physical properties of triple-negative breast cancers, which vary dynamically as the cancer progresses, profoundly impact the cancer's ability to spread," says Dr. Filipe. "These findings reveal a vulnerability of triple-negative breast cancers—the metastasizing cells' reliance on diverse fuel sources to meet their high energy demands."
Associate Professor Cox adds, "The study underscores the importance of considering the mechanical diversity within and between tumors when designing new treatments for aggressive cancers. We now plan to explore whether pairing targeted metabolic inhibitors with existing therapies could limit metastasis and improve outcomes for triple-negative breast cancer patients."
Explore further
Feedback to editors

Researchers demonstrate miniature brain stimulator in humans
11 hours ago
Study reveals potential to reverse lung fibrosis using the body's own healing technique
Apr 12, 2024
Researchers discover cell 'crosstalk' that triggers cancer cachexia

Study improves understanding of effects of household air pollution during pregnancy

Wearable sensors for Parkinson's can improve with machine learning, data from healthy adults

New insights on B cells: Researchers explore building better antibodies and curbing autoimmune diseases

Grieving pet owners comforted by 'supernatural' interactions

Study shows AI improves accuracy of skin cancer diagnoses

Cell's 'garbage disposal' may have another role: Helping neurons near skin sense the environment

Chlamydia vaccine shows promise in early trial
Related stories.
Researchers discover molecule that promotes production of cancer cells in triple-negative breast cancer
Mar 28, 2024
New drug halts growth of aggressive breast cancer in pre-clinical study
Feb 5, 2024
A new drug target for triple-negative breast cancer
Oct 17, 2022

Protein could offer therapeutic target for breast cancer metastasis
Feb 5, 2020

Removing microRNAs from triple-negative breast cancer cells can reverse its spread
Feb 5, 2021

Researchers uncover how cancer stem cells drive triple-negative breast cancer
Feb 8, 2018
Recommended for you
Scientists uncover a missing link between poor diet and higher cancer risk

Genomic deletions explain why some types of melanoma resist targeted therapies

Metformin may help the immune system better identify breast cancer cells

Presence of specific lipids indicate tissue aging and can be decreased through exercise, study shows
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Brief Communication
- Open access
- Published: 04 April 2024
Prognosis and treatment outcomes for patients with stage IA triple-negative breast cancer
- Paolo Tarantino ORCID: orcid.org/0000-0001-8686-0228 1 , 2 , 3 , 4 ,
- Julieta Leone 5 ,
- Carlos T. Vallejo 5 ,
- Rachel A. Freedman 1 , 2 , 3 ,
- Adrienne G. Waks 1 , 2 , 3 ,
- Olga Martínez-Sáez 1 , 2 , 6 , 7 ,
- Ana Garrido-Castro ORCID: orcid.org/0000-0002-5989-6636 1 , 2 , 3 ,
- Filipa Lynce ORCID: orcid.org/0000-0001-6615-7076 1 , 2 , 3 ,
- Nabihah Tayob 1 , 3 ,
- Nancy U. Lin 1 , 2 , 3 ,
- Sara M. Tolaney ORCID: orcid.org/0000-0002-5940-8671 1 , 2 , 3 &
- Jose P. Leone ORCID: orcid.org/0000-0002-8537-652X 1 , 2 , 3
npj Breast Cancer volume 10 , Article number: 26 ( 2024 ) Cite this article
1063 Accesses
33 Altmetric
Metrics details
- Breast cancer
To evaluate the role of chemotherapy in stage IA triple-negative breast cancer, we conducted a retrospective population-based study including 8601 patients. The use of chemotherapy significantly increased from 2010 to 2019 in patients with T1b and T1c tumors ( p = 0.001 and p < 0.001, respectively). Receipt of chemotherapy was associated with improved breast cancer-specific survival (BCSS, adjusted hazard ratio = 0.70; p = 0.006), particularly in patients with T1c tumors (5-year BCSS 94.5% vs. 91.2%).
Similar content being viewed by others
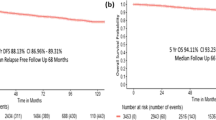
A multi-institutional real world data study from India of 3453 non-metastatic breast cancer patients undergoing upfront surgery
Dinesh Chandra Doval, Selvi Radhakrishna, … Ramesh B. V. Nimmagadda
Prolonged Time to Adjuvant Chemotherapy Initiation Was Associated with Worse Disease Outcome in Triple Negative Breast Cancer Patients
Lifen Cai, Yiwei Tong, … Xiaosong Chen
Biomarker dynamics and prognosis in breast cancer after neoadjuvant chemotherapy
Cristina Zarotti, Bärbel Papassotiropoulos, … Zsuzsanna Varga
Patients with early-stage triple-negative breast cancer (TNBC) have a higher risk of distant recurrence and death compared to patients with other breast cancer subtypes, including patients with small tumors 1 . Currently, adjuvant chemotherapy is the mainstay of systemic therapy for patients with stage I TNBC, who represent approximately one-third of TNBC patients.2 Among patients with stage IA TNBC (i.e., tumors ≤2 cm and node-negative), the National Comprehensive Cancer Network guidelines recommend adjuvant chemotherapy for tumors 1.1–2 cm (T1c), with consideration of adjuvant chemotherapy for tumors 0.6–1 cm (T1b). Patients with tumors ≤0.5 cm (T1a) are not recommended to receive adjuvant chemotherapy 2 , 3 . However, the utilization and benefit of chemotherapy for this population in the modern era remain poorly defined.
We conducted a population-based study using Surveillance, Epidemiology, and End Results (SEER) to investigate adjuvant chemotherapy treatment patterns and survival outcomes among patients diagnosed with stage IA TNBC between 2010 and 2019. We included 8601 women diagnosed with stage IA TNBC between 2010 and 2019. Patient demographics and disease information are included in Table 1 . The median age at diagnosis was 62 years old. Most patients (92.79%) had invasive ductal carcinomas, T1c disease (60.85%) and grade 3 (i.e., high-grade) tumors (70.14%). Adjuvant chemotherapy was administered to 5295 patients (61.6%). Median follow-up for survival analyses was 48 months (interquartile range: 20–83 months).
The rate of chemotherapy use among patients with T1mic and T1a tumors did not change significantly between 2010 and 2019 (<20% and <30% across years, respectively). However, chemotherapy use increased significantly from 2010-2019 among patients with T1b ( p = 0.001) and T1c tumors ( p < 0.0001), reaching ≥60% in patients with T1b and ≥70% in patients with T1c tumors across most years (Supplementary Table 1 , Supplementary Fig. 1 ).
In multivariable analyses, variables significantly associated with chemotherapy use (all p < 0.02) were younger age (age <50 vs. >64, odds ratio [OR] = 5.19), married status (married vs. single, OR = 1.28), high tumor grade (high grade [grade III] vs. low grade [grade I], OR = 4.89), and tumor size (Reference T1mic: T1a, OR = 2.91; T1b, OR = 19.16; T1c, OR = 31.49), among others (Supplementary Table 2 ).
Multivariable analyses revealed that chemotherapy receipt (vs. no/unknown receipt) was associated with improved BCSS among patients with stage IA TNBC. The 5-year BCSS for patients receiving chemotherapy vs. no/unknown was 95.2% vs. 94.4% (Adjusted hazard ratio [HR] = 0.70; 95% confidence interval [CI]: 0.55–0.90; Cox p value = 0.006) (Supplementary Fig. 2 ). When BCSS was evaluated by tumor size, patients with T1mic (Fig. 1a ) and T1a (Fig. 1b ) had excellent outcomes regardless of chemotherapy administration, with finding of only 1 (0.4%) and 17 (1.8%) breast cancer deaths, respectively (Supplementary Table 3 ). The small number of events in these two groups prevented adjusted comparisons. For those with T1b cancers, there was no significant association between chemotherapy and BCSS, with a total of 63 (2.9%) breast-cancer deaths observed (Fig. 1c ). In the setting of T1c disease, a total of 245 (4.7%) breast-cancer deaths were observed; in this subgroup, chemotherapy was associated with improved BCSS, with a 5-year BCSS of 94.5% for patients receiving chemotherapy vs. 91.2% in the no/unknown chemotherapy group (Adjusted HR = 0.64; 95% CI: 0.48–0.85; Cox p value = 0.002) (Fig. 1d ).
BCSS in a ) patients with T1mic disease; b ) patients with T1a disease; c ) patients with T1b disease; and d ) patients with T1c disease.
Our large population-based study revealed that women with stage IA TNBC have excellent 5-year BCSS outcomes, and that associations of chemotherapy receipt with survival were primarily observed in patients with T1c disease. Our findings are relatively consistent with similar studies conducted in this space, despite different sample sizes and methodologic differences. Vaz-Luis et al. 4 analyzed outcomes of patients with T1a-T1bN0 TNBC in the NCCN database ( n = 363), highlighting favorable 5-year BCSS ( ≥ 95%) in both T1a and T1b, irrespective of chemotherapy receipt. Studies conducted in SEER by refs. 5 , 6 , 7 showed improved outcomes among patients with T1c TNBC who received chemotherapy. In contrast to our study, the above studies included patients treated in the neoadjuvant setting, potentially including patients with occult nodal disease; we opted for excluding this population, so that we could ensure inclusion of patients with node-negative disease and provide more reliable estimates. Additionally, our study evaluated outcomes adjusted for histology, race, ethnicity, marital status, income, and rurality, which are relevant prognostic factors. A recent study by Carbajal-Ochoa evaluated only T1b and T1c TNBC and showed improved BCSS in T1c tumors 8 .
Our study adds to a body of evidence that suggests a benefit of adjuvant chemotherapy among stage IA TNBC patients with T1c tumors. Additionally, we observed an increase in chemotherapy use over time among patients with T1b and T1c tumors, possibly associated with increased reporting on recurrence rates for these tumors. Our study also provides important information on the favorable outcomes of patients with tumors ≤1 cm (T1mic, T1a, and T1b) that can inform discussions with patients. Indeed, the limited data available to treat small TNBCs has commonly led to extrapolation from studies of larger TNBCs for treating patients in clinical practice, with relevant risk for overtreatment and unnecessary toxicity. Important limitations of our study include its retrospective nature, with potential prognostic imbalances between subgroups, the absence of recurrence data in SEER and the lack of information on patient/clinician preferences and type of chemotherapies administered. Of note, the chemotherapy variable is coded in SEER as “no/unknown” when there was no evidence of chemotherapy administration, which prevents us from separating “no” from “unknown”.
In conclusion, in a large population-based study we observed excellent long-term outcomes for patients with stage IA TNBC and identified a progressive increase in the use of adjuvant chemotherapy for this population. An association between BCSS and use of adjuvant chemotherapy was identified for patients with T1c tumors, but not for T1b. Integration of additional prognostic factors and shared decision-making for treatment choices in this low-risk setting are warranted.
Data source and study design
We obtained data from SEER, using the 17 registries database (Nov. 2021 Submission). We extracted all cases of women diagnosed with stage IA TNBC (T1mic,a,b,c,N0,M0) from 2010 to 2019. To be included, patients must have had only one primary malignancy in their lifetime, had known vital status and cause of death ( n = 10,048). We excluded patients who did not undergo definitive breast surgery ( n = 314), those who received neoadjuvant chemotherapy ( n = 1116) or neoadjuvant radiation therapy ( n = 17) (Supplementary Fig. 3 ). The following variables were collected for analysis: age at diagnosis, race and ethnicity, marital status, median household income, rurality, year of diagnosis, histology, tumor grade, stage and size ( T ), type of surgery, receipt of adjuvant chemotherapy (coded as yes vs. no/unknown), receipt of adjuvant radiation therapy, vital status, and cause of death.
Statistical analyses
We examined the cohort characteristics and the frequency of chemotherapy use over time by tumor size. Variables associated with receipt of chemotherapy were evaluated using multivariate logistic regression. Nonparametric test for trend was used to evaluate the trend in chemotherapy use over time. We also examined breast cancer-specific survival (BCSS), defined as the interval from initial breast cancer diagnosis to death from breast cancer or last follow-up for censored patients. We used multivariable cox models to evaluate the association of adjuvant chemotherapy with BCSS stratified by tumor size, adjusted for age, race, ethnicity, tumor grade, histology, receipt of radiation therapy, marital status, income, and rurality. All p values were two-tailed and values <0.05 were considered statistically significant.
Ethical considerations
This study used de-identified, publicly available data. As such, it was exempt from review by the Dana-Farber Office for Human Research Studies.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Jose P. Leone had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are publicly available at SEER: https://seer.cancer.gov/ .
Lin, N. U. et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118 , 5463–5472, https://doi.org/10.1002/cncr.27581 (2012).
Article PubMed Google Scholar
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13 , 4429–4434, https://doi.org/10.1158/1078-0432.CCR-06-3045 (2007).
NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Guidelines Version 5.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Vaz-Luis, I. et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J. Clin. Oncol. 32 , 2142–2150, https://doi.org/10.1200/JCO.2013.53.1608 (2014).
Article PubMed PubMed Central Google Scholar
Du, Z. L. et al. Evaluation of a beneficial effect of adjuvant chemotherapy in patients with stage I triple-negative breast cancer: a population-based study using the SEER 18 database. Breast Cancer Res. Treat. 183 , 429–438, https://doi.org/10.1007/s10549-020-05776-2 (2020).
Article CAS PubMed Google Scholar
Zhai, Z. et al. Evaluation of adjuvant treatments for T1 N0 M0 triple-negative breast cancer. JAMA Netw. Open 3 , e2021881, https://doi.org/10.1001/jamanetworkopen.2020.21881 (2020).
Shen, K. et al. Impact of adjuvant chemotherapy on T1N0M0 breast cancer patients: a propensity score matching study based on SEER database and external cohort. BMC Cancer 22 , 863, https://doi.org/10.1186/s12885-022-09952-z (2022).
Article CAS PubMed PubMed Central Google Scholar
Carbajal-Ochoa, W., Bravo-Solarte, D. C., Bernal, A. M. & Anampa, J. D. Benefit of adjuvant chemotherapy in lymph node-negative, T1b and T1c triple-negative breast cancer. Breast Cancer Res. Treat. 203 , 257–269, https://doi.org/10.1007/s10549-023-07132-6 (2023).
Download references
Acknowledgements
This study received no funding. The authors acknowledge Timothy K. Erick for assistance in medical writing and Valerie Hope Goldstein for editorial assistance in the preparation of this manuscript. Both are full-time employees of Dana-Farber Cancer Institute.
Author information
Authors and affiliations.
Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA
Paolo Tarantino, Rachel A. Freedman, Adrienne G. Waks, Olga Martínez-Sáez, Ana Garrido-Castro, Filipa Lynce, Nabihah Tayob, Nancy U. Lin, Sara M. Tolaney & Jose P. Leone
Breast Oncology Program, Dana-Farber Brigham Cancer Center, Boston, MA, USA
Paolo Tarantino, Rachel A. Freedman, Adrienne G. Waks, Olga Martínez-Sáez, Ana Garrido-Castro, Filipa Lynce, Nancy U. Lin, Sara M. Tolaney & Jose P. Leone
Harvard Medical School, Boston, MA, USA
Paolo Tarantino, Rachel A. Freedman, Adrienne G. Waks, Ana Garrido-Castro, Filipa Lynce, Nabihah Tayob, Nancy U. Lin, Sara M. Tolaney & Jose P. Leone
Department of Oncology and Onco-Hematology, University of Milan, Milan, Italy
Paolo Tarantino
Grupo Oncológico Cooperativo del Sur, Neuquén, Argentina
Julieta Leone & Carlos T. Vallejo
Medical Oncology Department, Hospital Clinic of Barcelona, Barcelona, Spain
Olga Martínez-Sáez
Translational Genomics and Targeted Therapeutics in Solid Tumors, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Barcelona, Spain
You can also search for this author in PubMed Google Scholar
Contributions
P.T. helped with conceptualization, investigation, methodology, project administration, resources, software, supervision, validation, visualization, and writing—original draft, review and editing. J.L. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. C.T.V. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing-review and editing. R.A.F., A.G.W., O.M.-S., A.G.-C., F.L., N.U.L., and S.M.T. helped with investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. N.T. helped with data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. J.P.L. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, and writing-original draft, review and editing.
Corresponding author
Correspondence to Jose P. Leone .
Ethics declarations
Competing interests.
P.T. reports serving as advisor/consultant for AstraZeneca, Daiichi-Sankyo, Gilead and Lilly. O.M.S. reports travel expenses and consulting fees from Roche and Reveal Genomics and speaker fees from Eisai, Daiichi and Novartis. OMS is a SEOM visiting fellow 2022. A.G.C. reports research funding (to the Institution) from AstraZeneca, Daiichi-Sankyo, Merck, Gilead Sciences, Zenith Epigenetics, Bristol-Myers Squibb, Novartis, Biovica International AB, and Foundation Medicine; and travel/accommodations (to scientific meeting) from Roche/Genentech. R.A.F. reports institutional funding from Puma Biotechnology. F.L. reports consulting/advisory role for AstraZeneca, Pfizer, Merck and Daiichi-Sankyo; and institutional research funding from Eisai, AstraZeneca, CytomX and Gilead Sciences. N.U.L. reports institutional research support from Genentech, Pfizer, Merck, Seattle Genetics (now Pfizer), Zion Pharmaceuticals (as part of GNE), Olema Pharmaceuticals, and AstraZeneca; consulting honoraria from Puma, Seattle Genetics, Daiichi-Sankyo, AstraZeneca, Olema Pharmaceuticals, Janssen, Blueprint Medicines, Stemline/Menarini, and Artera Inc., and Eisai; royalties from UpToDate (book); and travel support from Olema Pharmaceuticals. SMT reports a consulting or advisory role for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi-Sankyo, Gilead, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Sumitovant Biopharma, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Biopharma, Bayer, Incyte Corp, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR, and Hengrui USA. S.M.T. receives research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, and OncoPep, and receives travel support from Eli Lilly, Sanofi, Gilead, and Pfizer. J.P.L. reports research funding from Kazia Therapeutics and consulting from Minerva Biotechnologies. The remaining authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary tables and figures, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tarantino, P., Leone, J., Vallejo, C.T. et al. Prognosis and treatment outcomes for patients with stage IA triple-negative breast cancer. npj Breast Cancer 10 , 26 (2024). https://doi.org/10.1038/s41523-024-00634-6
Download citation
Received : 19 December 2023
Accepted : 22 March 2024
Published : 04 April 2024
DOI : https://doi.org/10.1038/s41523-024-00634-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Search Please fill out this field.
- Manage Your Subscription
- Give a Gift Subscription
- Newsletters
- Sweepstakes
Mom Assumed Her Night Sweats Were a Symptom of Menopause — But She Had Cancer
Susete Ferreira initially thought she was experiencing typical symptoms of menopause including night sweats, a swollen abdomen, loss of appetite
:max_bytes(150000):strip_icc():format(webp)/IMG_4737-8b5b28d8b0e4469cbec8fe71e041ff12.jpeg)
Susete/Instagram
For months, Susete Ferreira thought she was experiencing the typical symptoms of menopause — night sweats, a swollen abdomen, loss of appetite. But suddenly she began experiencing an intense pain beneath her ribs.
Days later, she would be diagnosed with cancer.
"I started with a really sharp pain on my right side, under my ribs," Ferreira, 41, tells PEOPLE. "I thought it was maybe going to go away, but after four days of pain that would go away and come back, I went to the emergency room."
It was Sept. 2023 when Ferreira, who lives in Toronto, Ontario, says she first underwent an ultrasound, then a CT scan, which showed abnormalities in her lymph nodes. Two days after first being admitted to the ER, her gallbladder was removed and a biopsy was taken.
The test results came back as stage 4 follicular lymphoma.
"I was on my hospital bed and the oncologist came to my room and gave me the news," she tells PEOPLE. "I thought, I have to do something. I can't be the only one going through this. I felt very alone."
The stay-at-home mom decided to share her story on Instagram with the hope of connecting with other people facing difficult situations.
"Social media is such a powerful plug, I just felt sure I could find someone else going through something similar," she says.
Within two months, she had more than 100,000 followers, many of them inspired by her dancing videos from her hospital room.
"I couldn't believe the community I found on Instagram," says Ferreira, who has an 11-year-old son and 5-year-old daughter.
She also shares milestones in her cancer journey: In a recent video she rings the bell signifying the end of her first round of chemotherapy.
"Maintenance," as she calls it, will remain a crucial part of her health care, and Ferreira will undergo injections for immunotherapy every three months for the next two years.
For now, she urges her followers to get checked if they notice changes in their body — even if those changes seem like they might be part of the typical aging process.
Never miss a story — sign up for PEOPLE 's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from juicy celebrity news to compelling human interest stories.
"The night sweats are one thing to look out for. I had them for a long time and ignored them," she says. "Also a swollen abdomen. When I feel my belly bloating, it's not a good sign. It's a sign of inflammation and that's not good."
These days, Ferreira says she feels good, but continues to monitor her health and take things one day at a time.
"I feel like it's something that will always be a part of my life now," Ferreira says. "There are things you thought to be true before you had cancer, and there's what you think after ... everything changes. It gives you a different perspective in life, and forces you enjoy the smaller moments, the ones we normally take for granted."
Related Articles
- Entertainment
- KSAT Insider
- Newsletters
Woman who battled diabetes, cancer shares story of survival
Researchers have established a link between diabetes and cancers of the pancreas, liver, colon and breast.
Stephania Jimenez , Anchor
Rick Medina , Video Editor
SAN ANTONIO – You hear about diabetes all the time in South Texas. But did you know it’s linked to cancer?
According to UT Health San Antonio , 16% of San Antonians have diabetes, which is higher than the national rate of 10%. A 2021 study in the medical journal “Cancers” found up to 18% of cancer patients have diabetes.
For 54-year-old Virginia Cortes, it’s been a long journey to recovery.
“I was diagnosed with Type 2 diabetes (in) December of 2015, and I was diagnosed with breast cancer (in) September of 2019,” Cortes said.
In the last eight years, she’s lost weight, started diabetes medication and finished cancer treatment. As she continues to recover, she can’t help but wonder how she got here.
“Had I not had diabetes, could I have prevented the cancer? I don’t know,” said Cortes.
There’s no clear answer for Cortes. However, researchers have established a link between diabetes and cancers of the pancreas, liver, colon and breast.
“Diabetes is a chronic inflammatory disease that puts your body in override. That can lead abnormal cells to proliferate and then eventually cause cancer,” said Dr. Carolina Solis-Herrera, chief of endocrinology at UT Health San Antonio.
Solis-Herrera said diabetes and cancer patients can stay healthy by eating more vegetables, walking often and getting regular medical checkups.
“All those factors together will count to protect you from the complications (of) either heart attacks or strokes, blindness, amputation, or end-stage kidney disease, and then, of course, the cancers associated with diabetes,” Solis-Herrera said.
Now that Cortes is in remission, she told KSAT she sees light at the end of the tunnel. She hopes her experience inspires others to take care of their health.
“It’s preventable. It is hard to change, but you’ll change when you’re ready to change. And I hope it’s before you get diabetes,” said Solis-Herrera.
Copyright 2024 by KSAT - All rights reserved.
About the Authors
Stephania jimenez.
Stephania Jimenez is an anchor on The Nightbeat. She began her journalism career in 2006, after graduating from Syracuse University. She's anchored at NBC Philadelphia, KRIS in Corpus Christi, NBC Connecticut and KTSM in El Paso. Although born and raised in Brooklyn, Stephania considers Texas home. Stephania is bilingual! She speaks Spanish.
Rick Medina
Rick Medina is a Video News Editor at KSAT. A graduate of the University of Texas' prestigious Radio-Television-Film program, he has been in the news business for more than 20 years. Rick is also a documentary filmmaker, helming the award-winning film festival favorites, “The Opossum Begins” and “Amigoland.” He is originally from Brownsville.
Recommended Videos

Soledad twins, two-dozen cancer survivors take stage for 30th annual fashion show
T he American Cancer Society is hosting its annual Celebration of Life fashion show in Monterey Friday, marking the occasion with a special 30-year celebration.
The fashion show will be from 10:30 a.m. to 2:30 pm. Friday at the Monterey Marriott, 350 Calle Principal. All of the runway models are local cancer survivors who will be sharing their journey to inspire others and build community awareness. More than two dozen people are expected to take the stage.
Two of the women walking the runway this year are sisters from Soledad, who shared a cancer diagnosis and difficult recovery journey together. Fraternal twins Araceli and Angelica Villegas, 27, are sharing their story to inform people about early detection and listening to your body when you feel something is not right.
The journey started last year when Araceli was breastfeeding her son and became suspicious of a lump. When she later went to the doctor, advanced imaging confirmed she had breast cancer and the gene was genetic.
“The first thing I thought when I heard the word cancer is that I’m going to die,” Araceli Villegas said in a press release. “When they told me the BRAC gene was genetic, I knew my sister had to be tested as well.”
Though Angelica didn’t have any noticeable signs, her breast cancer turned out to be even more advanced. Both sisters received medical treatment at Salinas Valley Health Cancer Center of Excellence, which included chemotherapy, radiation, immunotherapy and a double mastectomy.
“The American Cancer Society has done so much to help support us and other cancer patients,” said Angelica Villegas in the press release. “We want to give back and hope to inspire others to support the cause and the important impact the Cancer Society has on the community, cancer research and patients like us.”
The 30-year celebration will also pay tribute to the founders of the fashion show, Shirley Lovorato, Karen Fanoe and Jeri Olivas in addition to honoring lives lost, celebrating other survivors and recognizing the impact the fundraiser has had in the community supporting cancer patients and their families.
Since its inception, the Celebration of Life event has raised more than $2 million through sponsors, local businesses and individual donations. The goal is to continue raising awareness and funding for those in treatment and survivors.
Although tickets are sold out for the event more information can be found at MtyFashionShow.GiveSmart.com.


IMAGES
VIDEO
COMMENTS
Meet Mindy, an emotional contributor at Learn Look Locate, who bravely shares her breast cancer journey to inspire and support others. Diagnosed with triple positive stage 2 invasive ductal carcinoma at 29, Mindy turned her battle into a beacon of hope for many facing similar challenges.
On July 10, 2016 Alene agreed to marry the love of her life. Just three days later, her entire world fell apart. Although she had no history of breast cancer in her family, she soon found herself sitting in a doctor's office trying to process the words nobody wants to hear. "You have breast cancer.". It all started with a routine mammogram.
Survival rate for women with stage 2 breast cancer is around 93% with treatment. Stage 2 breast cancer survival rates are high and with the right treatment, the outlook is very good. Stage 2 breast cancer means your tumor is at least 1 centimeter (cm) in size and has spread to lymph nodes.
Stage 2 breast cancer survival rate. The survival rate for stage 2A breast cancer may be slightly higher than for stage 2B. However, all patients with stage 2 breast cancer are considered to have a good prognosis. The five-year relative survival rate for stage 2A breast cancer patients is 98 percent, and for 2B, it's 95.6 percent, according to ...
The stages of breast cancer range from 0 to IV (0 to 4). The highest stage (stage IV) is any breast cancer with metastases (M1), no matter the size of the tumor, the lymph node status or other factors. This is known as metastatic breast cancer and is the most advanced stage of disease. Most often, the higher the stage of the cancer, the poorer ...
Stage 2 means the breast cancer is growing, but it is still contained in the breast or growth has only extended to the nearby lymph nodes. This stage is divided into groups: Stage 2A and Stage 2B. The difference is determined by the size of the tumor and whether the breast cancer has spread to the lymph nodes. For Stage 2 breast cancer ...
Stage 2 breast cancer is an early stage wherein the cancer has not spread beyond the breast and its surrounding lymph nodes. By some estimates, the average 5-year relative survival rate for stage ...
Stage 2 breast cancer consists of two subcategories, says Dr. Esteva. These are based on the size of the tumor and the degree to which it may have spread to nearby lymph nodes—small structures ...
Treatments. Surgery is standard. For smaller tumors, you might get a breast conserving surgery, or lumpectomy, in which only the tumor and some of the tissue around it are removed. For larger ...
Stages I-III. Treatment for stages I to III breast cancer usually includes surgery and radiation therapy, often with chemo or other drug therapies either before (neoadjuvant) or after (adjuvant) surgery. Stage I: These breast cancers are still relatively small and either have not spread to the lymph nodes or have only a tiny area of cancer ...
Targeted drug therapy: For women with early-stage breast cancer that is hormone receptor-positive, HER2-negative, has cancer in the lymph nodes, and has a high chance of coming back, the targeted drug abemaciclib can be given after surgery along with tamoxifen or an AI. It is a pill typically given for 2 years twice a day.
Breast cancer is the most common cancer and the one that associates the highest mortality rates among Spanish women, with 32,953 new cases estimated to be diagnosed in Spain in 2020 [ 1 ]. Thanks to early diagnosis and therapeutic advances, survival has increased in recent years [ 2 ]. The 5-year survival rate is currently around 85% [ 3, 4 ].
Jill Martin opens up after mastectomy: 'I want to share this journey in real-time' ... In the past six weeks, I learned I am positive for a BRCA2 mutation, had stage 2 breast cancer, then had ...
This patient was diagnosed with stage 2 breast cancer at age 41. Learn why she is urging other women to get mammograms and other breast cancer screening exams. ... Now, Sheila is devoting her energies to supporting other patients on their cancer journey by volunteering for Cancer Fighters®. Here, Sheila shares what she's learned about the ...
The earliest stage breast cancers are stage 0 (carcinoma in situ). It then ranges from stage I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage.
Stage IIIA is based on one of the following: With or without a tumor in the breast, cancer is found in four to nine nearby lymph nodes. A breast tumor is larger than 50 millimeters, and the cancer has spread to between one and three nearby lymph nodes. In stage IIIB, a tumor has spread to the chest wall behind the breast.
Triple-negative breast cancer (TNBC) is a very heterogeneous and aggressive breast cancer subtype with a high risk of mortality, even if diagnosed early. The mainstay of early-stage breast cancer includes systemic chemotherapy and surgery, with or without radiation therapy. More recently, immunotherapy is approved to treat TNBC, but managing immune-rated adverse events while balancing efficacy ...
Staging for breast cancer is very complex. Many different factors are considered before doctors can confirm your final stage. Speak to your doctor or breast cancer nurse specialist if you have any questions about your staging. Stage 2 can be divided into 2A and 2B. Below is a simplified description of stage 2A and 2B breast cancer. Stage 2A ...
The early symptoms can be harder to spot in males, and only 47% receive a diagnosis at this stage. As a result, the 5-year survival statistics for males are slightly lower than for females ...
Early-stage breast cancer is generally more treatable and is associated with a better prognosis compared with more advanced stages. Treatment options for early-stage breast cancer include surgery ...
In women who underwent axillary dissection and had one to three positive nodes (n = 1,314), radiotherapy reduced locoregional recurrence (2P < .00001), overall recurrence (2P = .00006), and breast cancer mortality (2P = .01), even in the setting of chemotherapy. Notably, the unirradiated women in these trials with one to three positive nodes ...
Eggert was diagnosed with stage 2 cribriform carcinoma breast cancer after discovering a lump in her breast while performing a self-breast exam. This type of exam is an easy way to keep watch for anything abnormal regarding your breasts. It involves feeling the breast for any swelling, bulging, or changes in the shape of the breast or nipple.
The study in cell and mouse models showed that softer tumor environments, typical of early-stage cancer, can prime triple-negative breast cancer cells to use an extra energy source for survival ...
To evaluate the role of chemotherapy in stage IA triple-negative breast cancer, we conducted a retrospective population-based study including 8601 patients. The use of chemotherapy significantly ...
Susete/Instagram. For months, Susete Ferreira thought she was experiencing the typical symptoms of menopause — night sweats, a swollen abdomen, loss of appetite. But suddenly she began ...
For 54-year-old Virginia Cortes, it's been a long journey to recovery. "I was diagnosed with Type 2 diabetes (in) December of 2015, and I was diagnosed with breast cancer (in) September of ...
Pain is different for every person with cancer. A plan to control pain needs to consider the person's diagnosis, type and stage of cancer, other health problems, medicines being taken, personal response to pain, and other personal choices. Pain can also be an early warning sign of the side effects of your cancer treatment or some other problem.
The fashion show will be from 10:30 a.m. to 2:30 pm. Friday at the Monterey Marriott, 350 Calle Principal. All of the runway models are local cancer survivors who will be sharing their journey to ...
Surgery is the first and most important line of treatment for nearly all patients with early-stage breast cancer, yet surgeons unfortunately lack tools to be more precise in their treatment planning decisions. ... This comprehensive approach aims to deliver holistic support for the cancer care journey, epitomizing the promise of true precision ...